Mechanisms of Spindle Positioning: Lessons from Worms and Mammalian Cells
- PMID: 30823600
- PMCID: PMC6406873
- DOI: 10.3390/biom9020080
Mechanisms of Spindle Positioning: Lessons from Worms and Mammalian Cells
Abstract
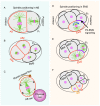
Proper positioning of the mitotic spindle is fundamental for specifying the site for cleavage furrow, and thus regulates the appropriate sizes and accurate distribution of the cell fate determinants in the resulting daughter cells during development and in the stem cells. The past couple of years have witnessed tremendous work accomplished in the area of spindle positioning, and this has led to the emergence of a working model unravelling in-depth mechanistic insight of the underlying process orchestrating spindle positioning. It is evident now that the correct positioning of the mitotic spindle is not only guided by the chemical cues (protein⁻protein interactions) but also influenced by the physical nature of the cellular environment. In metazoans, the key players that regulate proper spindle positioning are the actin-rich cell cortex and associated proteins, the ternary complex (Gα/GPR-1/2/LIN-5 in Caenorhabditis elegans, Gαi/Pins/Mud in Drosophila and Gαi1-3/LGN/NuMA in humans), minus-end-directed motor protein dynein and the cortical machinery containing myosin. In this review, I will mainly discuss how the abovementioned components precisely and spatiotemporally regulate spindle positioning by sensing the physicochemical environment for execution of flawless mitosis.
Keywords: NuMA; actin cytoskeleton; dynein; microtubules; mitosis; myosin; spindle positioning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Walczak C.E., Heald R. Mechanisms of mitotic spindle assembly and function. Int. Rev. Cytol. 2008;265:111–158. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

