Effects of a long-term home-based exercise training programme using minimal equipment vs. usual care in COPD patients: a study protocol for two multicentre randomised controlled trials (HOMEX-1 and HOMEX-2 trials)
- PMID: 30823913
- PMCID: PMC6397487
- DOI: 10.1186/s12890-019-0817-7
Effects of a long-term home-based exercise training programme using minimal equipment vs. usual care in COPD patients: a study protocol for two multicentre randomised controlled trials (HOMEX-1 and HOMEX-2 trials)
Abstract
Background: Exercise training is an important component of pulmonary rehabilitation (PR) programmes in chronic obstructive pulmonary disease (COPD), but the great majority of COPD patients who would benefit from PR never follow such programmes or fail to maintain exercise training after PR completion. Against this background, we developed an exercise training programme that requires minimal equipment and can be implemented long-term in the patient's home-setting. The aims of the HOMEX-1 and HOMEX-2 trials are to assess the effectiveness of this home-based exercise training programme in two groups of COPD patients over the course of one year: patients who have completed PR (HOMEX-1 trial) and patients who did not enrol in existing PR programmes within the last two years (HOMEX-2 trial).
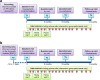
Methods: HOMEX-1 and HOMEX-2 are multicentre, parallel group, randomised controlled trials. For both trials each, it is planned to include 120 study participants with a diagnosis of COPD. Participants will be randomised with a 1:1 ratio into the intervention group or the control group (usual care/no intervention). The intervention consists of minimal-equipment exercise training elements with progressive level of intensity, conducted by the participant during six days per week and instructed and coached by a trained health care professional during three home visits and regular telephone calls during one year. Primary outcome is change in dyspnoea (domain of Chronic Respiratory Questionnaire) from baseline to 12-months follow-up. Secondary outcomes are change in dyspnoea over the course of the year (assessed at 3, 6 and 12 month) and change in functional exercise capacity, physical activity, health-related quality of life, health status, exacerbations and symptoms from baseline to 12 months follow-up. In addition, explanatory, safety and cost-effectiveness outcomes will be assessed. We will conduct intention-to-treat analyses separately per trial and per protocol analyses as sensitivity analyses.
Discussion: The HOMEX-1 and HOMEX-2 trials assess a novel intervention that provides an innovative way of making exercise training as accessible as possible for COPD patients. If the intervention proves to be effective long-term, it will fill the gap of providing an easily accessible and feasible intervention so that more COPD patients can follow an exercise programme.
Trial registration: ClinicalTrials.gov Identifier: HOMEX-1 NCT03461887 (registration date: March 12, 2018; retrospectively registered); HOMEX-2 NCT03654092 (registration date: August 31, 2018).
Keywords: COPD; Exercise training intervention; Functional exercise capacity; Home-based; Minimal equipment; Physical activity; Quality of life; Randomised controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
Both HOMEX trials were approved by the lead Ethics Committee Kantonale Ethikkommission Zürich, and by the local ethics committees Ethikkommission Bern and Ethikkommission Zentral- und Nordwestschweiz, Switzerland (BASEC-Nr. 2017–02092). All study participants will provide informed written consent before any study-related procedures are performed. The Ethics Committees will be informed about important protocol modifications for approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing or financial interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Sohanpal R, Steed L, Mars T, Taylor SJC. Understanding patient participation behaviour in studies of COPD support programmes such as pulmonary rehabilitation and self-management: a qualitative synthesis with application of theory. NPJ Prim Care Respir Med. 2015;25:15054. doi: 10.1038/npjpcrm.2015.54. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous