Molecular tumor analysis and liquid biopsy: a feasibility investigation analyzing circulating tumor DNA in patients with central nervous system lymphomas
- PMID: 30823914
- PMCID: PMC6397454
- DOI: 10.1186/s12885-019-5394-x
Molecular tumor analysis and liquid biopsy: a feasibility investigation analyzing circulating tumor DNA in patients with central nervous system lymphomas
Abstract
Background: Central nervous system lymphomas (CNSL) is a devastating disease. Currently, a confirmatory biopsy is required prior to treatment.
Objective: Our investigation aims to prove the feasibility of a minimally-invasive diagnostic approach for the molecular characterization of CNSL.
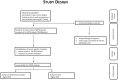
Methods: Tissue biopsies from 6 patients with suspected CNSL were analyzed using a 649gene next-generation sequencing (NGS) tumor panel (tumor vs. reference tissue (EDTA-blood)). The individual somatic mutation pattern was used as a basis for the digital PCR analyzing circulating tumor DNA (ctDNA) from plasma and cerebrospinal fluid (CSF) samples, identifying one selected tumor mutation during this first step of the feasibility investigation.
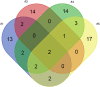
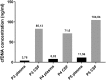
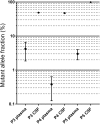
Results: NGS-analysis of biopsy tissue revealed a specific somatic mutation pattern in all confirmed lymphoma samples (n = 5, NGS-sensitivity 100%) and none in the sample identified as normal brain tissue (NGS-specificity 100%). cfDNA-extraction was dependent on the extraction-kit used and feasible in 3 samples, in all of which somatic mutations were detectable (100%). Analysis of CSF-derived cfDNA was superior to plasma-derived cfDNA and routine microscopic analysis (lymphoma cells: n = 2, 40%). One patient showed a divergent molecular pattern, typical of Burkitt-Lymphoma (HIV+, serologic evidence of EBV-infection). Lumbar puncture was tolerated without complications, whereas biopsy caused 3 hemorrhages.
Conclusions: Our investigation provides evidence that analysis of cfDNA in central nervous system tumors is feasible using the described protocol. Molecular characterization of CNSL could be achieved by analysis of CSF-derived cfDNA. Knowledge of a tumor's specific mutation pattern may allow initiation of targeted therapies, treatment surveillance and could lead to minimally-invasive diagnostics in the future.
Keywords: CNS-lymphoma; Cerebrospinal fluid; Circulating DNA; Liquid biopsy; Personalized medicine; Targeted therapies.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the local ethics committee (Ethikkommission der Landesärztekammer Baden-Württemberg, F-2010-030) and undertaken in accordance with national law, institutional ethical standards, and the Helsinki Declaration. Written informed consent was provided either by the patient or a legally competent next of kin prior to the first study specific intervention.
Consent for publication
As part of the written informed consent patients were informed about possible publication of the results of this investigation. Their consent to the study included a consent to publication of data while preserving anonymity. All presented data / images are selected without compromising the patients’ anonymity. Specific consent is not applicable.
Competing interests
Saskia Biskup is a co-founder of CeGaT GmbH (Tübingen, Germany). Dirk Hadaschik, Florian Battke and Dennis Döcker are currently employed by CeGaT GmbH (Tübingen, Germany) All other authors have no conflicts of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Detection of tumor-derived cell-free DNA in cerebrospinal fluid using a clinically validated targeted sequencing panel for pediatric brain tumors.J Neurooncol. 2024 Jun;168(2):215-224. doi: 10.1007/s11060-024-04645-y. Epub 2024 May 16. J Neurooncol. 2024. PMID: 38755519
-
Diagnosis of pediatric central nervous system tumors using methylation profiling of cfDNA from cerebrospinal fluid.Clin Epigenetics. 2024 Jul 5;16(1):87. doi: 10.1186/s13148-024-01696-w. Clin Epigenetics. 2024. PMID: 38970137 Free PMC article.
-
Heterogeneous mutation pattern in tumor tissue and circulating tumor DNA warrants parallel NGS panel testing.Mol Cancer. 2018 Aug 28;17(1):131. doi: 10.1186/s12943-018-0875-0. Mol Cancer. 2018. PMID: 30153823 Free PMC article.
-
Liquid biopsy for pediatric diffuse midline glioma: a review of circulating tumor DNA and cerebrospinal fluid tumor DNA.Neurosurg Focus. 2020 Jan 1;48(1):E9. doi: 10.3171/2019.9.FOCUS19699. Neurosurg Focus. 2020. PMID: 31896079 Free PMC article. Review.
-
Circulating Liquid Biopsy Biomarkers in Glioblastoma: Advances and Challenges.Int J Mol Sci. 2024 Jul 21;25(14):7974. doi: 10.3390/ijms25147974. Int J Mol Sci. 2024. PMID: 39063215 Free PMC article. Review.
Cited by
-
Clinical Value of ctDNA in Hematological Malignancies (Lymphomas, Multiple Myeloma, Myelodysplastic Syndrome, and Leukemia): A Meta-Analysis.Front Oncol. 2021 Mar 4;11:632910. doi: 10.3389/fonc.2021.632910. eCollection 2021. Front Oncol. 2021. PMID: 33747954 Free PMC article.
-
Liquid Biopsy in B and T Cell Lymphomas: From Bench to Bedside.Int J Mol Sci. 2025 May 19;26(10):4869. doi: 10.3390/ijms26104869. Int J Mol Sci. 2025. PMID: 40430009 Free PMC article. Review.
-
Detection of clonotypic DNA in the cerebrospinal fluid as a marker of central nervous system invasion in lymphoma.Blood Adv. 2021 Dec 28;5(24):5525-5535. doi: 10.1182/bloodadvances.2021004512. Blood Adv. 2021. PMID: 34551072 Free PMC article.
-
Prognostic value of pre-transplantation total metabolic tumor volume on 18fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography in relapsed and refractory aggressive lymphoma.Int J Hematol. 2022 Oct;116(4):603-611. doi: 10.1007/s12185-022-03394-w. Epub 2022 Jun 14. Int J Hematol. 2022. PMID: 35701707
-
Circulating tumor DNA in B-cell lymphoma: technical advances, clinical applications, and perspectives for translational research.Leukemia. 2022 Sep;36(9):2151-2164. doi: 10.1038/s41375-022-01618-w. Epub 2022 Jun 14. Leukemia. 2022. PMID: 35701522 Free PMC article. Review.
References
-
- Ferreri AJ, Marturano E. Primary CNS lymphoma. Best Pract Res Clin Haematol. 2012;25(1):119–130. - PubMed
-
- Feiden W, Milutinovic S. Primary CNS lymphomas. Morphology and diagnosis. Pathologe. 2002;23(4):284–291. - PubMed
-
- Benesova L, Belsanova B, Suchanek S, Kopeckova M, Minarikova P, Lipska L, Levy M, Visokai V, Zavoral M, Minarik M. Mutation-based detection and monitoring of cell-free tumor DNA in peripheral blood of cancer patients. Anal Biochem. 2013;433(2):227–234. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

