Nanobody-horseradish peroxidase fusion protein as an ultrasensitive probe to detect antibodies against Newcastle disease virus in the immunoassay
- PMID: 30823927
- PMCID: PMC6396497
- DOI: 10.1186/s12951-019-0468-0
Nanobody-horseradish peroxidase fusion protein as an ultrasensitive probe to detect antibodies against Newcastle disease virus in the immunoassay
Abstract
Background: Sensitive and specific antibodies can be used as essential probes to develop competitive enzyme-linked immunosorbent assay (cELISA). However, traditional antibodies are difficult to produce, only available in limited quantities, and ineffective as enzymatic labels. Nanobodies, which are single-domain antibodies (sdAbs), offer an alternative, more promising tool to circumvent these limitations. In the present work, a cELISA using nanobody-horseradish peroxidase (HRP) fusion protein firstly designed as a probe was developed for detecting anti-Newcastle disease virus (NDV) antibodies in chicken sera.
Results: In the study, a platform for the rapid and simple production of nanobody-HRP fusion protein was constructed. First, a total of 9 anti-NDV-NP protein nanobodies were screened from a immunised Bactrian camel. Then, the Nb5-HRP fusions were produced with the platform and used for the first time as sensitive reagents for developing cELISA to detect anti-NDV antibodies. The cut-off value of the cELISA was 18%, and the sensitivity and specificity were respectively 100% and 98.6%. The HI test and commercial ELISA kit (IDEXX) separately agreed 97.83% and 98.1% with cELISA when testing clinical chicken sera and both agreed 100% when testing egg yolks. However, for detecting anti-NDV antibodies in the sequential sera from the challenged chickens, cELISA demonstrated to be more sensitive than the HI test and commercial ELISA kit. Moreover, a close correlation (R2 = 0.914) was found between the percent competitive inhibition values of cELISA and HI titers.
Conclusions: A platform was successfully designed to easily and rapidly produce the nanobody-HRP fusion protein, which was the first time to be used as reagents for establishing cELISA. Results suggest that the platform supports the development of a cELISA with high sensitivity, simplicity, and rapid detection of anti-NDV antibodies. Overall, we believe that the platform based on nanobody-HRP fusions can be widely used for future investigations and treatment other diseases and viruses.
Keywords: Antibody; Competitive ELISA; NDV; Nanobody; Nanobody-HRP.
Figures





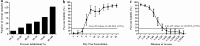


References
-
- Teillaud JL. From whole monoclonal antibodies to single domain antibodies: think small. Methods Mol Biol. 2012;911:3–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

