A conserved and buried edge-to-face aromatic interaction in small ubiquitin-like modifier (SUMO) has a role in SUMO stability and function
- PMID: 30824543
- PMCID: PMC6497963
- DOI: 10.1074/jbc.RA118.006642
A conserved and buried edge-to-face aromatic interaction in small ubiquitin-like modifier (SUMO) has a role in SUMO stability and function
Abstract
Aromatic amino acids buried at a protein's core are often involved in mutual paired interactions. Ab initio energy calculations have highlighted that the conformational orientations and the effects of substitutions are important for stable aromatic interactions among aromatic rings, but studies in the context of a protein's fold and function are elusive. Small ubiquitin-like modifier (SUMO) is a common post-translational modifier that affects diverse cellular processes. Here, we report that a highly conserved aromatic triad of three amino acids, Phe36-Tyr51-Phe64, is a unique SUMO signature that is absent in other ubiquitin-like homologous folds. We found that a specific edge-to-face conformation between the Tyr51-Phe64 pair of interacting aromatics is vital to the fold and stability of SUMO. Moreover, the noncovalent binding of SUMO-interacting motif (SIM) at the SUMO surface was critically dependent on the paired aromatic interactions buried at the core. NMR structural studies revealed that perturbation of the Tyr51-Phe64 conformation disrupts several long-range tertiary contacts in SUMO, leading to a heterogeneous and dynamic protein with attenuated SUMOylation both in vitro and in cells. A subtle perturbation of the edge-to-face conformation by a Tyr to Phe substitution significantly decreased stability, SUMO/SIM affinity, and the rate of SUMOylation. Our results highlight that absolute co-conservation of specific aromatic pairs inside the SUMO protein core has a role in its stability and function.
Keywords: SUMO-interacting motif (SIM); aromatic interactions; aromatic ring; aromatic triad; nuclear magnetic resonance (NMR); post-translational modification (PTM); protein dynamics; protein misfolding; protein structure; sumoylation.
© 2019 Chatterjee et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

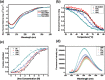


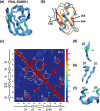
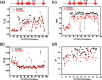

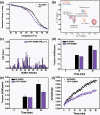
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

