The Origin of Biological Homochirality
- PMID: 30824575
- PMCID: PMC6396334
- DOI: 10.1101/cshperspect.a032540
The Origin of Biological Homochirality
Abstract
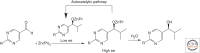
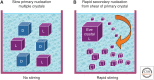
The fact that sugars, amino acids, and the biological polymers they construct exist exclusively in one of two possible mirror-image forms has fascinated scientists and laymen alike for more than a century. Yet, it was only in the late 20th century that experimental studies began to probe how biological homochirality, a signature of life, arose from a prebiotic world that presumably contained equal amounts of both mirror-image forms of these molecules. This review discusses experimental studies aimed at understanding how chemical reactions, physical processes, or a combination of both may provide prebiotically relevant mechanisms for the enrichment of one form of a chiral molecule over the other to allow for the emergence of biological homochirality.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures






References
-
- Blackmond DG, McMillan CR, Ramdeehul S, Schorm A, Brown JM. 2001. Origins of asymmetric amplification in autocatalytic alkylzinc additions. J Am Chem Soc 123: 10103–10104. - PubMed
-
- Breslow R. 1959. On the mechanism of the formose reaction. Tetrahedron Lett 22–36.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
