Quantitative proteomic analyses of dynamic signalling events in cortical neurons undergoing excitotoxic cell death
- PMID: 30824683
- PMCID: PMC6397184
- DOI: 10.1038/s41419-019-1445-0
Quantitative proteomic analyses of dynamic signalling events in cortical neurons undergoing excitotoxic cell death
Abstract
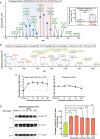
Excitotoxicity, caused by overstimulation or dysregulation of ionotropic glutamate receptors (iGluRs), is a pathological process directing neuronal death in many neurological disorders. The aberrantly stimulated iGluRs direct massive influx of calcium ions into the affected neurons, leading to changes in expression and phosphorylation of specific proteins to modulate their functions and direct their participation in the signalling pathways that induce excitotoxic neuronal death. To define these pathways, we used quantitative proteomic approaches to identify these neuronal proteins (referred to as the changed proteins) and determine how their expression and/or phosphorylation dynamically changed in association with excitotoxic cell death. Our data, available in ProteomeXchange with identifier PXD008353, identified over 100 changed proteins exhibiting significant alterations in abundance and/or phosphorylation levels at different time points (5-240 min) in neurons after glutamate overstimulation. Bioinformatic analyses predicted that many of them are components of signalling networks directing defective neuronal morphology and functions. Among them, the well-known neuronal survival regulators including mitogen-activated protein kinases Erk1/2, glycogen synthase kinase 3 (GSK3) and microtubule-associated protein (Tau), were selected for validation by biochemical approaches, which confirmed the findings of the proteomic analysis. Bioinformatic analysis predicted Protein Kinase B (Akt), c-Jun kinase (JNK), cyclin-dependent protein kinase 5 (Cdk5), MAP kinase kinase (MEK), Casein kinase 2 (CK2), Rho-activated protein kinase (Rock) and Serum/glucocorticoid-regulated kinase 1 (SGK1) as the potential upstream kinases phosphorylating some of the changed proteins. Further biochemical investigation confirmed the predictions of sustained changes of the activation states of neuronal Akt and CK2 in excitotoxicity. Thus, future investigation to define the signalling pathways directing the dynamic alterations in abundance and phosphorylation of the identified changed neuronal proteins will help elucidate the molecular mechanism of neuronal death in excitotoxicity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

