Anti-VEGF injection frequency correlates with visual acuity outcomes in pro re nata neovascular AMD treatment
- PMID: 30824721
- PMCID: PMC6397189
- DOI: 10.1038/s41598-019-38934-8
Anti-VEGF injection frequency correlates with visual acuity outcomes in pro re nata neovascular AMD treatment
Abstract
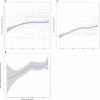
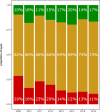
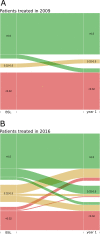
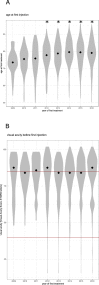
Clinical trials report substantial gains in visual acuity (VA) for eyes treated with intravitreal anti-VEGF for neovascular AMD (nAMD). In clinical reality, VA outcomes are more variable. Here we investigate pro-re nata treatment frequencies and VA in a real-life cohort of 1382 eyes (1048 patients). Patients with nAMD and one year complete follow-up treated with pro-re nata anti-VEGF between 2009 and 2016 were included. Injection frequency and VA was analyzed clustered by year of first treatment. Baseline parameters were compared between years. Median injection frequency in the first year was 5 with an IQR (interquartile range) of 5 for patients treated in 2009 and 8 with an IQR of 3 for patients treated from 2012 onwards. Median VA outcomes at one year were -5 to ±0 letters for patients treated between 2009 and 2013 and ±0 to +2 letters for patients treated from 2013 onwards. This cohort comprises all severities and subtypes of nAMD. 39% of patients had baseline VA outside the range for the MARINA or ANCHOR clinical trials. Higher treatment frequency was associated with improved VA in our real-life nAMD cohort. With adequate injection frequency, almost 90% of eyes had stable or improved VA over one year. Median VA gains, however, were lower compared to clinical trials. This may be due to a wider range of baseline characteristics in real-life cohorts.
Conflict of interest statement
T.W. has received speaker fees from Novartis. H.A. has received research funds from Novartis as well as honoraria/speaker fees/advisory fees from Roche, Allergan, Zeiss, Bayer Healthcare. A.S. has received research funds from Novartis Germany as well as honoraria/speaker fees/advisory fees from Allergan, Bayer, Boehringer Ingelheim, Novartis, Orphan Europe. The other authors declare no competing interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

