Development of T cell immunity to norovirus and rotavirus in children under five years of age
- PMID: 30824789
- PMCID: PMC6397277
- DOI: 10.1038/s41598-019-39840-9
Development of T cell immunity to norovirus and rotavirus in children under five years of age
Abstract
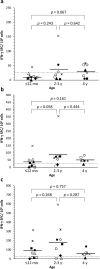
Most of the research effort to understand protective immunity against norovirus (NoV) has focused on humoral immunity, whereas immunity against another major pediatric enteric virus, rotavirus (RV), has been studied more thoroughly. The aim of this study was to investigate development of cell-mediated immunity to NoV in early childhood. Immune responses to NoV GI.3 and GII.4 virus-like particles and RV VP6 were determined in longitudinal blood samples of 10 healthy children from three months to four years of age. Serum IgG antibodies were measured using enzyme-linked immunosorbent assay and production of interferon-gamma by peripheral blood T cells was analyzed by enzyme-linked immunospot assay. NoV-specific T cells were detected in eight of 10 children by the age of four, with some individual variation. T cell responses to NoV GII.4 were higher than those to GI.3, but these responses were generally lower than responses to RV VP6. In contrast to NoV-specific antibodies, T cell responses were transient in nature. No correlation between cell-mediated and antibody responses was observed. NoV exposure induces vigorous T cell responses in children under five years of age, similar to RV. A role of T cells in protection from NoV infection in early childhood warrants further investigation.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

