Immune cells as targets for cardioprotection: new players and novel therapeutic opportunities
- PMID: 30825305
- PMCID: PMC6529904
- DOI: 10.1093/cvr/cvz050
Immune cells as targets for cardioprotection: new players and novel therapeutic opportunities
Abstract
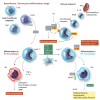
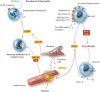
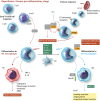
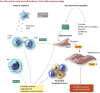
New therapies are required to reduce myocardial infarct (MI) size and prevent the onset of heart failure in patients presenting with acute myocardial infarction (AMI), one of the leading causes of death and disability globally. In this regard, the immune cell response to AMI, which comprises an initial pro-inflammatory reaction followed by an anti-inflammatory phase, contributes to final MI size and post-AMI remodelling [changes in left ventricular (LV) size and function]. The transition between these two phases is critical in this regard, with a persistent and severe pro-inflammatory reaction leading to adverse LV remodelling and increased propensity for developing heart failure. In this review article, we provide an overview of the immune cells involved in orchestrating the complex and dynamic inflammatory response to AMI-these include neutrophils, monocytes/macrophages, and emerging players such as dendritic cells, lymphocytes, pericardial lymphoid cells, endothelial cells, and cardiac fibroblasts. We discuss potential reasons for past failures of anti-inflammatory cardioprotective therapies, and highlight new treatment targets for modulating the immune cell response to AMI, as a potential therapeutic strategy to improve clinical outcomes in AMI patients. This article is part of a Cardiovascular Research Spotlight Issue entitled 'Cardioprotection Beyond the Cardiomyocyte', and emerged as part of the discussions of the European Union (EU)-CARDIOPROTECTION Cooperation in Science and Technology (COST) Action, CA16225.
Keywords: Acute myocardial infarction; Dendritic cells; Fibroblasts; Inflammation; Lymphocytes; Macrophages; Monocytes; Myocardial ischaemia/reperfusion injury.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2019. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, James S, Kellerth T, Lindahl B, Ravn-Fischer A, Rydberg E, Yndigegn T, Jernberg T.. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995-2014. Eur Heart J 2017;38:3056–3065. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

