Dual-isotope imaging allows in vivo immunohistochemistry using radiolabelled antibodies in tumours
- PMID: 30825614
- PMCID: PMC6599172
- DOI: 10.1016/j.nucmedbio.2019.01.010
Dual-isotope imaging allows in vivo immunohistochemistry using radiolabelled antibodies in tumours
Abstract
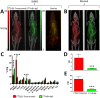
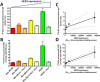
While radiolabelled antibodies have found great utility as PET and SPECT imaging agents in oncological investigations, a notable shortcoming of these agents is their propensity to accumulate non-specifically within tumour tissue. The degree of this non-specific contribution to overall tumour uptake is highly variable and can ultimately lead to false conclusions. Therefore, in an effort to obtain a reliable measure of inter-individual differences in non-specific tumour uptake of radiolabelled antibodies, we demonstrate that the use of dual-isotope imaging overcomes this issue, enables true quantification of epitope expression levels, and allows non-invasive in vivo immunohistochemistry. The approach involves co-administration of (i) an antigen-targeting antibody labelled with zirconium-89 (89Zr), and (ii) an isotype-matched non-specific control IgG antibody labelled with indium-111 (111In). As an example, the anti-HER2 antibody trastuzumab was radiolabelled with 89Zr, and co-administered intravenously together with its 111In-labelled non-specific counterpart to mice bearing human breast cancer xenografts with differing HER2 expression levels (MDA-MB-468 [HER2-negative], MDA-MB-231 [low-HER2], MDA-MB-231/H2N [medium-HER2], and SKBR3 [high-HER2]). Simultaneous PET/SPECT imaging using a MILabs Vector4 small animal scanner revealed stark differences in the intratumoural distribution of [89Zr]Zr-trastuzumab and [111In]In-IgG, highlighting regions of HER2-mediated uptake and non-specific uptake, respectively. Normalisation of the tumour uptake values and tumour-to-blood ratios obtained with [89Zr]Zr-trastuzumab against those obtained with [111In]In-IgG yielded values which were most strongly correlated (R = 0.94; P = 0.02) with HER2 expression levels for each breast cancer type determined by Western blot and in vitro saturation binding assays, but not non-normalised uptake values. Normalised intratumoural distribution of [89Zr]Zr-trastuzumab correlated well with intratumoural heterogeneity HER2 expression.
Keywords: Antibody; Dual-isotope; HER2; Molecular imaging; PET; SPECT.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Mestel R. Cancer: imaging with antibodies. Nature. 2017;543:743. - PubMed
-
- Gebhart G., Lamberts L.E., Wimana Z., Garcia C., Emonts P., Ameye L. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27:619–624. - PubMed
-
- Lamberts L.E., Williams S.P., Terwisscha van Scheltinga A.G.T., Lub-de Hooge M.N., Schröder C.P., Gietema J.A. Antibody positron emission tomography imaging in anticancer drug development. J Clin Oncol. 2015;33:1491–1504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

