Use of (meth)acrylamides as alternative monomers in dental adhesive systems
- PMID: 30826074
- PMCID: PMC6462417
- DOI: 10.1016/j.dental.2019.02.012
Use of (meth)acrylamides as alternative monomers in dental adhesive systems
Abstract
Objectives: Methacrylamides are proposed as components for dental adhesive systems with enhanced resistance to hydrolytic and enzymatic degradation. The specific objective of this study was to evaluate the polymerization kinetics, water sorption and solubility, pH-derived degradation and microtensile bond strength of various monofunctional acrylamides and meth(acrylamides) when copolymerized with dimethacrylates.
Methods: Base monomers were added at 60 wt%, and included either BisGMA or UDMA. Monofunctional monomers were added at 40 wt%, including one (meth)acrylate as the control, two secondary methacrylamides and two tertiary acrylamides. DMPA (0.2 wt%) and DPI-PF6 (0.4 wt%)/BHT (0.1 wt%) were added as initiators/inhibitor. Polymerization kinetics wwere followed with near-IR spectroscopy in real time. Water sorption (WS) and solubility (SL) were measured following ISO 4049. Monomer degradation at different pH levels was assessed with 1H NMR. Microtensile bond strength (MTBS) was assessed in caries-free human third molars 48 h and 3 weeks after restorations were placed using solvated BisGMA-based adhesives (40 vol% ethanol). Data were analyzed with one-way ANOVA/Tukey's test (α = 0.05).
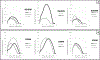
Results: As expected, rate of polymerization and final degree of conversion (DC) were higher for the acryl versions of each monomer, and decreased with increasing steric hindrance around the vinyl group for each molecule. In general, UDMA copolymerizations were more rapid and extensive than for BisGMA, but this was dependent upon the specific monofunctional monomer added. WS/SL were in general higher for the (meth)acrylamides compared to the (meth)acrylates, except for the tertiary acrylamide, which showed the lowest values. One of the secondary methacrylamides was significantly more stable than the methacrylate control, but the alpha substitutions decreased stability to degradation in acid pH. MTBS in general was higher for the (meth)acrylates. While for all materials the MTBS values at 3 weeks decreased in relation to the 24 h results, the tertiary acrylamide showed no reduction in bond strength.
Significance: This study highlights the importance of considering steric and electronic factors when designing monomers for applications where rapid polymerizations are needed, especially when co-polymerizations with other base monomers are required to balance mechanical properties, as is the case with dental adhesives. The results of this investigation will be used to design fully formulated adhesives to be tested in clinically-relevant conditions.
Keywords: Copolymerization; Methacrylamides; Phase-separation; Polymer network; Polymerization kinetics; Steric hindrance.
Copyright © 2019 The Academy of Dental Materials. Published by Elsevier Inc. All rights reserved.
Figures

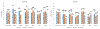


References
-
- Nakabayashi N, Takarada K. Effect of HEMA on bonding to dentin. Dental Materials. 1992;8:125–30. - PubMed
-
- Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85. - PubMed
-
- Burrow MF, Inokoshi S, Tagami J. Water sorption of several bonding resins. American Journal of Dentistry. 1999;12:295–8. - PubMed
-
- Tanaka J, Ishikawa K, Yatani H, Yamashita A, Suzuki K. Correlation of dentin bond durability with water absorption of bonding layer. Dent Mater J. 1999;18:11–8. - PubMed
-
- Tay FR, Pashley DH. Water treeing - A potential mechanism for degradation of dentin adhesives. American Journal of Dentistry. 2003;16:6–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

