The role of the mobile proton in fucose migration
- PMID: 30826852
- PMCID: PMC6611747
- DOI: 10.1007/s00216-019-01657-w
The role of the mobile proton in fucose migration
Abstract
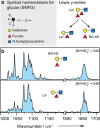
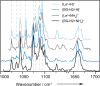
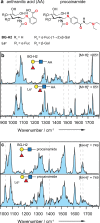
Fucose migration reactions represent a substantial challenge in the analysis of fucosylated glycan structures by mass spectrometry. In addition to the well-established observation of transposed fucose residues in glycan-dissociation product ions, recent experiments show that the rearrangement can also occur in intact glycan ions. These results suggest a low-energy barrier for migration of the fucose residue and broaden the relevance of fucose migration to include other types of mass spectrometry experiments, including ion mobility-mass spectrometry and ion spectroscopy. In this work, we utilize cold-ion infrared spectroscopy to provide further insight into glycan scrambling in intact glycan ions. Our results show that the mobility of the proton is a prerequisite for the migration reaction. For the prototypical fucosylated glycans Lewis x and blood group antigen H-2, the formation of adduct ions or the addition of functional groups with variable proton affinity yields significant differences in the infrared spectra. These changes correlate well with the promotion or inhibition of fucose migration through the presence or absence of a mobile proton.
Keywords: Carbohydrates; Fucose migration; Infrared spectroscopy; Internal residue loss; Mass spectrometry.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Mass spectrometry of proton adducts of fucosylated N-glycans: fucose transfer between antennae gives rise to misleading fragments.Rapid Commun Mass Spectrom. 2006;20(11):1747-54. doi: 10.1002/rcm.2509. Rapid Commun Mass Spectrom. 2006. PMID: 16676317
-
Fucose Migration in Intact Protonated Glycan Ions: A Universal Phenomenon in Mass Spectrometry.Angew Chem Int Ed Engl. 2018 Jun 18;57(25):7440-7443. doi: 10.1002/anie.201801418. Epub 2018 May 25. Angew Chem Int Ed Engl. 2018. PMID: 29688603
-
Fucose Migration Pathways Identified Using Infrared Spectroscopy.Angew Chem Int Ed Engl. 2023 Apr 17;62(17):e202300538. doi: 10.1002/anie.202300538. Epub 2023 Mar 14. Angew Chem Int Ed Engl. 2023. PMID: 36825496
-
Glycan analysis by ion mobility-mass spectrometry and gas-phase spectroscopy.Curr Opin Chem Biol. 2018 Feb;42:16-24. doi: 10.1016/j.cbpa.2017.10.021. Epub 2017 Nov 5. Curr Opin Chem Biol. 2018. PMID: 29107930 Review.
-
Fucose: biosynthesis and biological function in mammals.Glycobiology. 2003 Jul;13(7):41R-53R. doi: 10.1093/glycob/cwg054. Epub 2003 Mar 19. Glycobiology. 2003. PMID: 12651883 Review.
Cited by
-
Linkage Memory in Underivatized Protonated Carbohydrates.J Am Soc Mass Spectrom. 2021 Feb 3;32(2):581-589. doi: 10.1021/jasms.0c00440. Epub 2020 Dec 22. J Am Soc Mass Spectrom. 2021. PMID: 33350817 Free PMC article.
-
Towards Mapping of the Human Brain N-Glycome with Standardized Graphitic Carbon Chromatography.Biomolecules. 2022 Jan 6;12(1):85. doi: 10.3390/biom12010085. Biomolecules. 2022. PMID: 35053234 Free PMC article.
-
New strategies for profiling and characterization of human milk oligosaccharides.Glycobiology. 2020 Sep 28;30(10):774-786. doi: 10.1093/glycob/cwaa028. Glycobiology. 2020. PMID: 32248230 Free PMC article.
-
Evidence of Gas Phase Glucosyl Transfer and Glycation in the CID/HCD-Spectra of S-Glucosylated Peptides.Int J Mol Sci. 2024 Jul 8;25(13):7483. doi: 10.3390/ijms25137483. Int J Mol Sci. 2024. PMID: 39000590 Free PMC article.
-
Ion mobility-tandem mass spectrometry of mucin-type O-glycans.Nat Commun. 2024 Mar 23;15(1):2611. doi: 10.1038/s41467-024-46825-4. Nat Commun. 2024. PMID: 38521783 Free PMC article.
References
-
- Dwek RA. Glycobiology: toward understanding the function of sugars. Chem Rev. 1996;96(2):683–720. - PubMed
-
- Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126(5):841–845. - PubMed
-
- Myers RB, Srivastava S, Grizzle WE. Lewis Y antigen as detected by the monoclonal antibody BR96 is expressed strongly in prostatic adenocarcinoma. J Urol. 153(5):1572–4. - PubMed
-
- Werz DB, Ranzinger R, Herget S, Adibekian A, von der Lieth C-W, Seeberger PH. Exploring the structural diversity of mammalian carbohydrates (“glycospace”) by statistical databank analysis. ACS Chem Biol. 2007;2(10):685–691. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

