Phosphorylation of Syntaxin 17 by TBK1 Controls Autophagy Initiation
- PMID: 30827897
- PMCID: PMC6907693
- DOI: 10.1016/j.devcel.2019.01.027
Phosphorylation of Syntaxin 17 by TBK1 Controls Autophagy Initiation
Abstract
Syntaxin 17 (Stx17) has been implicated in autophagosome-lysosome fusion. Here, we report that Stx17 functions in assembly of protein complexes during autophagy initiation. Stx17 is phosphorylated by TBK1 whereby phospho-Stx17 controls the formation of the ATG13+FIP200+ mammalian pre-autophagosomal structure (mPAS) in response to induction of autophagy. TBK1 phosphorylates Stx17 at S202. During autophagy induction, Stx17pS202 transfers from the Golgi, where its steady-state pools localize, to the ATG13+FIP200+ mPAS. Stx17pS202 was in complexes with ATG13 and FIP200, whereas its non-phosphorylatable mutant Stx17S202A was not. Stx17 or TBK1 knockouts blocked ATG13 and FIP200 puncta formation. Stx17 or TBK1 knockouts reduced the formation of ATG13 protein complexes with FIP200 and ULK1. Endogenous Stx17pS202 colocalized with LC3B following induction of autophagy. Stx17 knockout diminished LC3 response and reduced sequestration of the prototypical bulk autophagy cargo lactate dehydrogenase. We conclude that Stx17 is a TBK1 substrate and that together they orchestrate assembly of mPAS.
Keywords: TBK1; ULK1; autophagy; pre-autophagosomal structure.
Published by Elsevier Inc.
Conflict of interest statement
Figures


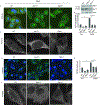
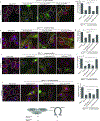

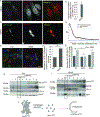

References
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/B/000C0413/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 AI042999/AI/NIAID NIH HHS/United States
- BB/K019155/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- P20 GM121176/GM/NIGMS NIH HHS/United States
- R37 AI042999/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

