Anti-PEG Antibodies Inhibit the Anticoagulant Activity of PEGylated Aptamers
- PMID: 30827937
- PMCID: PMC6707742
- DOI: 10.1016/j.chembiol.2019.02.001
Anti-PEG Antibodies Inhibit the Anticoagulant Activity of PEGylated Aptamers
Abstract
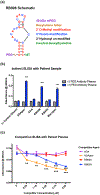
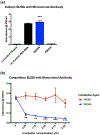
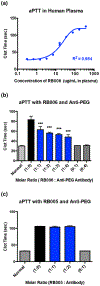
Biopharmaceuticals have become increasingly attractive therapeutic agents and are often PEGylated to enhance their pharmacokinetics and reduce their immunogenicity. However, recent human clinical trials have demonstrated that administration of PEGylated compounds can evoke anti-PEG antibodies. Considering the ubiquity of PEG in commercial products and the presence of pre-existing anti-PEG antibodies in patients in large clinical trials evaluating a PEG-modified aptamer, we investigated how anti-PEG antibodies effect the therapeutic activities of PEGylated RNA aptamers. We demonstrate that anti-PEG antibodies can directly bind to and inhibit anticoagulant aptamer function in vitro and in vivo. Moreover, in parallel studies we detected the presence of anti-PEG antibodies in nonhuman primates after a single administration of a PEGylated aptamer. Our results suggest that anti-PEG antibodies can limit the activity of PEGylated drugs and potentially compromise the activity of otherwise effective therapeutic agents.
Keywords: ELISA (enzyme-linked immunosorbent assay); PEG (polyethylene glycol); PEGylation; RB006; REGULATE-PCI; aPTT (activated partial thromboplastin time); anti-PEG antibodies; aptamer; hypersensitivity; rhesus monkeys.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests:
The authors have declared no conflict of interest exists. However, Duke University has submitted patent applications on the anticoagulant aptamers.
Figures
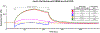
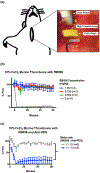
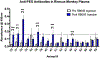
Comment in
-
To PEGylate or Not To PEGylate Therapeutics?Cell Chem Biol. 2019 May 16;26(5):615-616. doi: 10.1016/j.chembiol.2019.04.014. Cell Chem Biol. 2019. PMID: 31100258
References
-
- Aberle JH, Aberle SW, Redlberger-Fritz M, Sandhofer MJ, and Popow-Kraupp T (2010). Human metapneumovirus subgroup changes and seasonality during epidemics. Pediatr Infect Dis J 29, 1016–1018. - PubMed
-
- Abuchowski A, McCoy JR, Palczuk NC, van Es T, and Davis FF (1977a). Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem 252, 3582–3586. - PubMed
-
- Abuchowski A, van Es T, Palczuk NC, and Davis FF (1977b). Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem 252, 3578–3581. - PubMed
-
- Chan MY, Cohen MG, Dyke CK, Myles SK, Aberle LG, Lin M., Walder J, Steinhubl SR, Gilchrist IC, Kleiman NS, et al. (2008). Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation 117, 2865–2874. - PubMed
-
- Cohen MG, Purdy DA, Rossi JS, Grinfeld LR, Myles SK, Aberle LH, Greenbaum AB, Fry E, Chan MY, Tonkens RM, et al. (2010). First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous coronary intervention. Circulation 122, 614–622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

