Estrogens Modulate Somatostatin Receptors Expression and Synergize With the Somatostatin Analog Pasireotide in Prostate Cells
- PMID: 30828298
- PMCID: PMC6384260
- DOI: 10.3389/fphar.2019.00028
Estrogens Modulate Somatostatin Receptors Expression and Synergize With the Somatostatin Analog Pasireotide in Prostate Cells
Erratum in
-
Corrigendum: Estrogens modulate somatostatin receptors expression and synergize with the somatostatin analog pasireotide in prostate cells.Front Pharmacol. 2024 Dec 2;15:1515349. doi: 10.3389/fphar.2024.1515349. eCollection 2024. Front Pharmacol. 2024. PMID: 39687300 Free PMC article.
Abstract
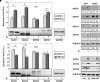
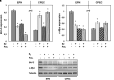
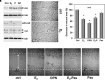
Prostate cancer (PC) is one of the most frequently diagnosed cancers and a leading cause of cancer-related deaths in Western society. Current PC therapies prevalently target the functions of androgen receptor (AR) and may only be effective within short time periods, beyond which the majority of PC patients progress to castration-resistant PC (CRPC) and metastatic disease. The role of estradiol/estradiol receptor (ER) axis in prostate transformation and PC progression is well established. Further, considerable efforts have been made to investigate the mechanism by which somatostatin (SST) and somatostatin receptors (SSTRs) influence PC growth and progression. A number of therapeutic strategies, such as the combination of SST analogs with other drugs, show, indeed, strong promise. However, the effect of the combined treatment of SST analogs and estradiol on proliferation, epithelial mesenchyme transition (EMT) and migration of normal- and cancer-derived prostate cells has not been investigated so far. We now report that estradiol plays anti-proliferative and pro-apoptotic effect in non-transformed EPN prostate cells, which express both ERα and ERβ. A weak apoptotic effect is observed in transformed CPEC cells that only express low levels of ERβ. Estradiol increases, mainly through ERα activation, the expression of SSTRs in EPN, but not CPEC cells. As such, the hormone enhances the anti-proliferative effect of the SST analog, pasireotide in EPN, but not CPEC cells. Estradiol does not induce EMT and the motility of EPN cells, while it promotes EMT and migration of CPEC cells. Addition of pasireotide does not significantly modify these responses. Altogether, our results suggest that pasireotide may be used, alone or in combination with other drugs, to limit the growth of prostate proliferative diseases, provided that both ER isoforms (α and β) are present. Further investigations are needed to better define the cross talk between estrogens and SSTRs as well as its role in PC.
Keywords: EMT; apoptosis; estrogens; migration; prostate cancer; somatostatin analogs; somatostatin receptors.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Estrogen Receptors Promote Migration, Invasion and Colony Formation of the Androgen-Independent Prostate Cancer Cells PC-3 Through β-Catenin Pathway.Front Endocrinol (Lausanne). 2020 Apr 9;11:184. doi: 10.3389/fendo.2020.00184. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32328032 Free PMC article.
-
Raloxifene induces cell death and inhibits proliferation through multiple signaling pathways in prostate cancer cells expressing different levels of estrogen receptor α and β.J Cell Physiol. 2011 May;226(5):1334-9. doi: 10.1002/jcp.22461. J Cell Physiol. 2011. PMID: 20945400
-
Somatostatin, Cortistatin and Their Receptors Exert Antitumor Actions in Androgen-Independent Prostate Cancer Cells: Critical Role of Endogenous Cortistatin.Int J Mol Sci. 2022 Oct 27;23(21):13003. doi: 10.3390/ijms232113003. Int J Mol Sci. 2022. PMID: 36361790 Free PMC article.
-
Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications.Front Oncol. 2018 Jan 18;8:2. doi: 10.3389/fonc.2018.00002. eCollection 2018. Front Oncol. 2018. PMID: 29404276 Free PMC article. Review.
-
Estrogen receptor signaling in prostate cancer: Implications for carcinogenesis and tumor progression.Prostate. 2018 Jan;78(1):2-10. doi: 10.1002/pros.23446. Epub 2017 Nov 2. Prostate. 2018. PMID: 29094395 Review.
Cited by
-
Signal Crosstalk and the Role of Estrogen Receptor beta (ERβ) in Prostate Cancer.Med Sci Monit. 2022 Apr 6;28:e935599. doi: 10.12659/MSM.935599. Med Sci Monit. 2022. PMID: 35383138 Free PMC article. Review.
-
Nerve Growth Factor Induces Proliferation and Aggressiveness In Prostate Cancer Cells.Cancers (Basel). 2019 Jun 6;11(6):784. doi: 10.3390/cancers11060784. Cancers (Basel). 2019. PMID: 31174415 Free PMC article.
-
SIRT7 depletion inhibits cell proliferation and androgen-induced autophagy by suppressing the AR signaling in prostate cancer.J Exp Clin Cancer Res. 2020 Feb 4;39(1):28. doi: 10.1186/s13046-019-1516-1. J Exp Clin Cancer Res. 2020. PMID: 32019578 Free PMC article.
-
Estrogen Receptors Promote Migration, Invasion and Colony Formation of the Androgen-Independent Prostate Cancer Cells PC-3 Through β-Catenin Pathway.Front Endocrinol (Lausanne). 2020 Apr 9;11:184. doi: 10.3389/fendo.2020.00184. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32328032 Free PMC article.
-
Utilization of macrocyclic peptides to target protein-protein interactions in cancer.Front Oncol. 2022 Nov 17;12:992171. doi: 10.3389/fonc.2022.992171. eCollection 2022. Front Oncol. 2022. PMID: 36465350 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

