Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes
- PMID: 30828317
- PMCID: PMC6384237
- DOI: 10.3389/fendo.2019.00080
Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes
Erratum in
-
Corrigendum: Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes.Front Endocrinol (Lausanne). 2019 May 3;10:275. doi: 10.3389/fendo.2019.00275. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31133983 Free PMC article.
Abstract
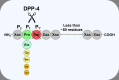
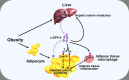
Dipeptidyl peptidase-4 (DPP-4), also known as the T-cell antigen CD26, is a multi-functional protein which, besides its catalytic activity, also functions as a binding protein and a ligand for a variety of extracellular molecules. It is an integral membrane protein expressed on cells throughout the body, but is also shed from the membrane and circulates as a soluble protein in the plasma. A large number of bioactive molecules can be cleaved by DPP-4 in vitro, but only a few of these have been demonstrated to be physiological substrates. One of these is the incretin hormone, glucagon-like peptide-1 (GLP-1), which plays an important role in the maintenance of normal glucose homeostasis, and DPP-4 has been shown to be the key enzyme regulating its biological activity. This pathway has been targeted pharmacologically through the development of DPP-4 inhibitors, and these are now a successful class of anti-hyperglycaemic agents used to treat type 2 diabetes (T2DM). DPP-4 may additionally influence metabolic control via its proteolytic effect on other regulatory peptides, but it has also been reported to affect insulin sensitivity, potentially mediated through its non-enzymatic interactions with other membrane proteins. Given that altered expression and activity of DPP-4 are associated with increasing body mass index and hyperglycaemia, DPP-4 has been proposed to play a role in linking obesity and the pathogenesis of T2DM by functioning as a local mediator of inflammation and insulin resistance in adipose and hepatic tissue. As well as these broader systemic effects, it has also been suggested that DPP-4 may be able to modulate β-cell function as part of a paracrine system involving GLP-1 produced locally within the pancreatic islets. However, while it is evident that DPP-4 has the potential to influence glycaemic control, its overall significance for the normal physiological regulation of glucose homeostasis in humans and its role in the pathogenesis of metabolic disease remain to be established.
Keywords: dipeptidyl peptidase-4; glucagon-like peptide-1; incretin; peptide degradation; therapy; type 2 diabetes.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

