Gut Microbiome as Target for Innovative Strategies Against Food Allergy
- PMID: 30828329
- PMCID: PMC6384262
- DOI: 10.3389/fimmu.2019.00191
Gut Microbiome as Target for Innovative Strategies Against Food Allergy
Abstract
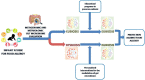
The dramatic increase in food allergy prevalence and severity globally requires effective strategies. Food allergy derives from a defect in immune tolerance mechanisms. Immune tolerance is modulated by gut microbiota function and structure, and microbiome alterations (dysbiosis) have a pivotal role in the development of food allergy. Environmental factors, including a low-fiber/high-fat diet, cesarean delivery, antiseptic agents, lack of breastfeeding, and drugs can induce gut microbiome dysbiosis, and have been associated with food allergy. New experimental tools and technologies have provided information regarding the role of metabolites generated from dietary nutrients and selected probiotic strains that could act on immune tolerance mechanisms. The mechanisms are multiple and still not completely defined. Increasing evidence has provided useful information on optimal bacterial species/strains, dosage, and timing for intervention. The increased knowledge of the crucial role played by nutrients and gut microbiota-derived metabolites is opening the way to a post-biotic approach in the stimulation of immune tolerance through epigenetic regulation. This review focused on the potential role of gut microbiome as the target for innovative strategies against food allergy.
Keywords: butyrate; dysbiosis; gut microbiota; gut microbiota metabolites; immune tolerance; mediterranean diet; probiotics; short chain fatty acids.
Figures



References
-
- McBride D, Keil T, Grabenhenrich L, Dubakiene R, Drasutiene G, Fiocchi A, et al. The EuroPrevall birth cohort study on food allergy: baseline characteristics of 12,000 newborns and their families from nine European countries. Pediatr Allergy Immunol. (2012) 23:230–9. 10.1111/j.1399-3038.2011.01254.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

