A comprehensive assessment of inbreeding and laboratory adaptation in Aedes aegypti mosquitoes
- PMID: 30828375
- PMCID: PMC6383739
- DOI: 10.1111/eva.12740
A comprehensive assessment of inbreeding and laboratory adaptation in Aedes aegypti mosquitoes
Abstract
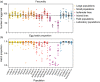
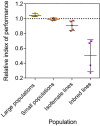
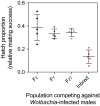
Modified Aedes aegypti mosquitoes reared in laboratories are being released around the world to control wild mosquito populations and the diseases they transmit. Several efforts have failed due to poor competitiveness of the released mosquitoes. We hypothesized that colonized mosquito populations could suffer from inbreeding depression and adapt to laboratory conditions, reducing their performance in the field. We established replicate populations of Ae. aegypti mosquitoes collected from Queensland, Australia, and maintained them in the laboratory for twelve generations at different census sizes. Mosquito colonies maintained at small census sizes (≤100 individuals) suffered from inbreeding depression due to low effective population sizes which were only 25% of the census size as estimated by SNP markers. Populations that underwent full-sib mating for nine consecutive generations had greatly reduced performance across all traits measured. We compared the established laboratory populations with their ancestral population resurrected from quiescent eggs for evidence of laboratory adaptation. The overall performance of laboratory populations maintained at a large census size (400 individuals) increased, potentially reflecting adaptation to artificial rearing conditions. However, most individual traits were unaffected, and patterns of adaptation were not consistent across populations. Differences between replicate populations may indicate that founder effects and drift affect experimental outcomes. Though we find limited evidence of laboratory adaptation, mosquitoes maintained at low population sizes can clearly suffer fitness costs, compromising the success of "rear-and-release" strategies for arbovirus control.
Keywords: Aedes aegypti; biological control; colonization; inbreeding; laboratory adaptation.
Figures



References
-
- Allgood, D. W. , & Yee, D. A. (2014). Influence of resource levels, organic compounds and laboratory colonization on interspecific competition between the Asian tiger mosquito Aedes albopictus (Stegomyia albopicta) and the southern house mosquito Culex quinquefasciatus . Medical and Veterinary Entomology, 28(3), 273–286. - PMC - PubMed
-
- Andrews, S. (2010). FastQC: a quality control tool for high throughput sequence data. Retrieved from https://www.bioinformatics.babraham.ac.uk/projects/fastqc
-
- Axford, J. K. , Ross, P. A. , Yeap, H. L. , Callahan, A. G. , & Hoffmann, A. A. (2016). Fitness of wAlbB Wolbachia infection in Aedes aegypti: Parameter estimates in an outcrossed background and potential for population invasion. American Journal of Tropical Medicine and Hygiene, 94(3), 507–516. 10.4269/ajtmh.15-0608 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

