Père David's deer gut microbiome changes across captive and translocated populations: Implications for conservation
- PMID: 30828378
- PMCID: PMC6383733
- DOI: 10.1111/eva.12743
Père David's deer gut microbiome changes across captive and translocated populations: Implications for conservation
Abstract
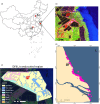
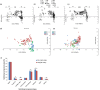
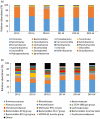
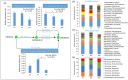
The gut microbial composition and function are shaped by different factors (e.g., host diet and phylogeny). Gut microbes play an important role in host nutrition and development. The gut microbiome may be used to evaluate the host potential environmental adaptation. In this study, we focused on the coevolution of the gut microbiome of captive and translocated Père David's deer populations (Elaphurus davidianus; Chinese: Père David's deer). To address this, we used several different macro- and micro-ecological approaches (landscape ecology, nutritional methods, microscopy, isotopic analysis, and metagenomics). In this long-term study (2011-2014), we observed some dissimilarities in gut microbiome community and function between the captive and wild/translocated Dafeng Père David's deer populations. These differences might link microbiome composition with deer diet within a given season. The proportion of genes coding for putative enzymes (endoglucanase, beta-glucosidase, and cellulose 1,4-beta-cellobiosidase) involved in cellulose digestion in the gut microbiome of the captive populations was higher than that of the translocated population, possibly because of the high proportion of cellulose, hemicellulose, and lignin in the plants most consumed by the captive populations. However, the two enzymes (natA and natB) involved in sodium transport system were enriched in the gut microbiome in translocated population, possibly because of their high salt diet (e.g., Spartina alterniflora). Thus, our results suggested that Père David's deer gut microorganisms potentially coevolved with host diet, and reflected the local adaptation of translocated population in the new environment (e.g., new dietary plants: Spartina alterniflora). A current problem for Père David's deer conservation is the saturation of captive populations. Given that the putative evolutionary adaptation of Père David's deer gut microbiome and its possible applications in conservation, the large area of wetlands along the Yellow Sea dominated by S. alterniflora might be the major translocation region in the future.
Keywords: Père David’s deer; coevolution; conservation; gut microbiome; sodium transport system; translocated populations.
Figures


Comment in
-
Evaluation of the Function of Wild Animal Gut Microbiomes Using Next-Generation Sequencing and Bioinformatics and its Relevance to Animal Conservation.Evol Bioinform Online. 2019 May 10;15:1176934319848438. doi: 10.1177/1176934319848438. eCollection 2019. Evol Bioinform Online. 2019. PMID: 31205409 Free PMC article.
References
-
- Breiman, L. (2001). Random forests. Machine Learning, 45(1), 5–32.
-
- Cao, K. (1985). On the reasons of extinction of the wild mi‐deer in China. Zoological Research, 6(1), 111–115.
Associated data
LinkOut - more resources
Full Text Sources

