Trial design and baseline data for LIRA-PRIME: A randomized trial investigating the efficacy of liraglutide in controlling glycaemia in type 2 diabetes in a primary care setting
- PMID: 30828917
- PMCID: PMC6617804
- DOI: 10.1111/dom.13682
Trial design and baseline data for LIRA-PRIME: A randomized trial investigating the efficacy of liraglutide in controlling glycaemia in type 2 diabetes in a primary care setting
Abstract
Aims: Using a pragmatic approach, the LIRA-PRIME trial aims to address a knowledge gap by comparing efficacy in controlling glycaemia with glucagon-like peptide-1 analog liraglutide vs oral antidiabetic drugs (OADs) in patients with type 2 diabetes (T2D) uncontrolled with metformin monotherapy in primary care practice. We report the study design and patient baseline characteristics.
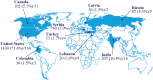
Materials and methods: This 104-week, two-arm, open-label, active-controlled trial is active in 219 primary care practices across nine countries. At screening, eligible patients with T2D were at least 18 years of age, had been using a stable daily dose of metformin ≥1500 mg or the maximum tolerated dose for ≥60 days, and had a glycated haemoglobin (HbA1c) of 7.5% to 9.0%, measured ≤90 days before screening. Patients were randomized (1:1) to liraglutide or OAD, both in addition to pre-trial metformin. Individual OADs were chosen by the treating physician based on local guidelines. The primary endpoint is time to inadequate glycaemic control, defined as HbA1c above 7.0% at two scheduled consecutive visits after the first 26 weeks of treatment.
Results: The trial randomized 1997 patients with a mean (standard deviation) age of 56.9 (10.8) years, T2D duration of 7.2 (5.9) years (range, <1-47 years), and HbA1c of 8.2%. One-fifth of patients had a history of diabetes complications, and most were overweight (24.8%) or had obesity (65.3%).
Conclusions: This pragmatically designed, large-scale, multinational, randomized clinical trial will help guide treatment decisions for patients with T2D who are inadequately controlled with metformin monotherapy and treated in primary care.
Keywords: GLP-1; liraglutide; type 2 diabetes.
© 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Author contributions
J. U. conducted follow‐up assessments of patients and oversaw the study. D. C. A. conducted follow‐up assessments of patients. M. C. conducted follow‐up assessments of patients. K. L. conducted screening and follow‐up assessments of patients. D. L. conducted follow‐up assessments of patients. G. M. conducted follow‐up assessments of patients. J. K. P. contributed to study design and data management. M. S. conducted follow‐up assessments of patients. M. K. contributed to trial design, medical oversight during trial conduct, data cleaning and analysis and interpretation of the data. M. B. T. contributed to analysis and interpretation of the data. M. Z. conducted follow‐up assessments of patients. All authors interpreted the data and participated in writing the manuscript with the assistance of medical writing services provided by the trial sponsor. All authors read the manuscript critically and approved the submitted article prior to submission.
Figures

References
-
- American Diabetes Association . 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S73‐S85. - PubMed
-
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577‐1589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

