An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program
- PMID: 30828977
- PMCID: PMC6516180
- DOI: 10.1111/acel.12933
An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program
Abstract
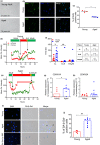
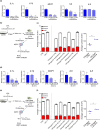
Hematopoietic stem and progenitor cells (HSPC) reside in the bone marrow (BM) niche and serve as a reservoir for mature blood cells throughout life. Aging in the BM is characterized by low-grade chronic inflammation that could contribute to the reduced functionality of aged HSPC. Mesenchymal stromal cells (MSC) in the BM support HSPC self-renewal. However, changes in MSC function with age and the crosstalk between MSC and HSPC remain understudied. Here, we conducted an extensive characterization of senescence features in BM-derived MSC from young and aged healthy donors. Aged MSC displayed an enlarged senescent-like morphology, a delayed clonogenic potential and reduced proliferation ability when compared to younger counterparts. Of note, the observed proliferation delay was associated with increased levels of SA-β-galactosidase (SA-β-Gal) and lipofuscin in aged MSC at early passages and a modest but consistent accumulation of physical DNA damage and DNA damage response (DDR) activation. Consistent with the establishment of a senescence-like state in aged MSC, we detected an increase in pro-inflammatory senescence-associated secretory phenotype (SASP) factors, both at the transcript and protein levels. Conversely, the immunomodulatory properties of aged MSC were significantly reduced. Importantly, exposure of young HSPC to factors secreted by aged MSC induced pro-inflammatory genes in HSPC and impaired HSPC clonogenic potential in a SASP-dependent manner. Altogether, our results reveal that BM-derived MSC from aged healthy donors display features of senescence and that, during aging, MSC-associated secretomes contribute to activate an inflammatory transcriptional program in HSPC that may ultimately impair their functionality.
Keywords: DNA damage; SASP; aging; hematopoietic stem and progenitor cells; inflammation; mesenchymal stromal cells; senescence.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None Declared.
Figures




Similar articles
-
Aging-Related Reduced Expression of CXCR4 on Bone Marrow Mesenchymal Stromal Cells Contributes to Hematopoietic Stem and Progenitor Cell Defects.Stem Cell Rev Rep. 2020 Aug;16(4):684-692. doi: 10.1007/s12015-020-09974-9. Stem Cell Rev Rep. 2020. PMID: 32418119 Free PMC article.
-
Quantifying Senescence-Associated Phenotypes in Primary Multipotent Mesenchymal Stromal Cell Cultures.Methods Mol Biol. 2019;2045:93-105. doi: 10.1007/7651_2019_217. Methods Mol Biol. 2019. PMID: 31020633
-
Increased IL-6 secretion by aged human mesenchymal stromal cells disrupts hematopoietic stem and progenitor cells' homeostasis.Oncotarget. 2016 Mar 22;7(12):13285-96. doi: 10.18632/oncotarget.7690. Oncotarget. 2016. PMID: 26934440 Free PMC article.
-
Hematopoietic Niche - Exploring Biomimetic Cues to Improve the Functionality of Hematopoietic Stem/Progenitor Cells.Biotechnol J. 2018 Feb;13(2). doi: 10.1002/biot.201700088. Epub 2017 Dec 28. Biotechnol J. 2018. PMID: 29178199 Review.
-
[Research Advance of on the Support Effect of Adipose Tissue-Derived Stem Cell on Hematopoietic Stem/Progenitor Cell--Review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021 Feb;29(1):301-305. doi: 10.19746/j.cnki.issn.1009-2137.2021.01.051. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021. PMID: 33554839 Review. Chinese.
Cited by
-
Comparable in vitro Function of Human Liver-Derived and Adipose Tissue-Derived Mesenchymal Stromal Cells: Implications for Cell-Based Therapy.Front Cell Dev Biol. 2021 Mar 26;9:641792. doi: 10.3389/fcell.2021.641792. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33842466 Free PMC article.
-
The functional role of cellular senescence during vascular calcification in chronic kidney disease.Front Endocrinol (Lausanne). 2024 Jan 22;15:1330942. doi: 10.3389/fendo.2024.1330942. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38318291 Free PMC article. Review.
-
Paracrine Senescence of Mesenchymal Stromal Cells Involves Inflammatory Cytokines and the NF-κB Pathway.Cells. 2022 Oct 21;11(20):3324. doi: 10.3390/cells11203324. Cells. 2022. PMID: 36291189 Free PMC article.
-
Aged mesenchymal stem cells and inflammation: from pathology to potential therapeutic strategies.Biol Direct. 2023 Jul 18;18(1):40. doi: 10.1186/s13062-023-00394-6. Biol Direct. 2023. PMID: 37464416 Free PMC article. Review.
-
Roles of Microenvironment on Mesenchymal Stem Cells Therapy for Osteoarthritis.J Inflamm Res. 2024 Oct 3;17:7069-7079. doi: 10.2147/JIR.S475617. eCollection 2024. J Inflamm Res. 2024. PMID: 39377043 Free PMC article. Review.
References
-
- Avanzini, M. A. , Bernardo, M. E. , Cometa, A. M. , Perotti, C. , Zaffaroni, N. , Novara, F. , … Locatelli, F. (2009). Generation of mesenchymal stromal cells in the presence of platelet lysate: A phenotypic and functional comparison of umbilical cord blood‐ and bone marrow‐ derived progenitors. Haematologica, 94(12), 1649–1660. 10.3324/haematol.2009.006171 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

