Cell-mediated immune responses to different formulations of a live-attenuated tetravalent dengue vaccine candidate in subjects living in dengue endemic and non-endemic regions
- PMID: 30829100
- PMCID: PMC6773406
- DOI: 10.1080/21645515.2019.1581536
Cell-mediated immune responses to different formulations of a live-attenuated tetravalent dengue vaccine candidate in subjects living in dengue endemic and non-endemic regions
Abstract
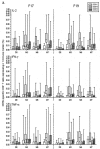
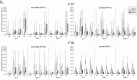
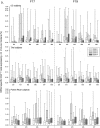
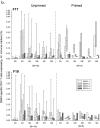
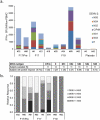
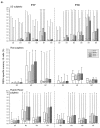
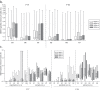
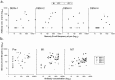
Three phase II randomized trials evaluated the safety/immunogenicity of two formulations of live-attenuated tetravalent dengue virus (TDEN) vaccine in dengue-endemic (Puerto Rico, Thailand) and non-endemic (US) regions (NCT00350337/NCT00370682/NCT00468858). We describe cell-mediated immune (CMI) responses; safety and humoral responses were reported previously. Participants received two doses of vaccine or control (placebo or the precursor live-attenuated TDEN vaccine) 6 months apart. Selected US participants received a booster 5-12 months post-dose 2. Evaluated subsets of the per-protocol cohorts included 75 primarily dengue virus (DENV)-unprimed US adults, 69 primarily flavivirus-primed Thai adults, and 100 DENV-primed or DENV-unprimed Puerto Rican adults/adolescents/children. T-cell responses were quantified using intracellular cytokine staining (ICS; DENV-infected cell-lysate or DENV-1/DENV-2 peptide-pool stimulation) or IFN-γ ELISPOT (DENV-2 peptide-pool stimulation). Memory B-cell responses were quantified using B-cell ELISPOT. Across populations and age strata, DENV serotype-specific CD4+ T-cell responses were slightly to moderately increased (medians ≤0.18% [ICS]), DENV-2-biased, and variable for both formulations. Responses in unprimed subjects were primarily detected post-dose 1. Response magnitudes in primed subjects were similar between doses. Multifunctional CD8+ T-cell responses were detected after peptide-pool stimulation. T-cell responses were mostly directed to DENV nonstructural proteins 3 and 5. Memory B-cell responses were tetravalent, of low-to-moderate magnitudes (medians ≤0.25%), and mainly observed post-dose 2 in unprimed subjects and post-dose 1 in primed subjects. A third dose did not boost CMI responses. In conclusion, both formulations of the live-attenuated TDEN vaccine candidate were poorly to moderately immunogenic with respect to B-cell and T-cell responses, irrespective of the priming status of the participants. Abbreviation ATP: according-to-protocol; ICS: Intracellular Cytokine Staining; NS3: Nonstructural protein 3; ELISPOT: Enzyme-Linked ImmunoSpot; JEV: Japanese encephalitis virus; PBMC: peripheral blood mononuclear cells.
Keywords: Cellular-mediated immune responses; Dengue-primed and -unprimed populations; Live-attenuated tetravalent dengue candidate vaccine.
Figures


References
-
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castaneda-Orjuela CA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(6):712–23. doi: 10.1016/S1473-3099(16)00026-8. - DOI - PMC - PubMed
-
- WHO Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. Switzerland: World Health Organization; 1997.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
