Extended Treatment with Glial Cell Line-Derived Neurotrophic Factor in Parkinson's Disease
- PMID: 30829619
- PMCID: PMC6597995
- DOI: 10.3233/JPD-191576
Extended Treatment with Glial Cell Line-Derived Neurotrophic Factor in Parkinson's Disease
Abstract
Background: Intraputamenal glial cell line-derived neurotrophic factor (GDNF), administered every 4 weeks to patients with moderately advanced Parkinson's disease, did not show significant clinical improvements against placebo at 40 weeks, although it significantly increased [18F]DOPA uptake throughout the entire putamen.
Objective: This open-label extension study explored the effects of continued (prior GDNF patients) or new (prior placebo patients) exposure to GDNF for another 40 weeks.
Methods: Using the infusion protocol of the parent study, all patients received GDNF without disclosing prior treatment allocations (GDNF or placebo). The primary outcome was the percentage change from baseline to Week 80 in the OFF state Unified Parkinson's Disease Rating Scale (UPDRS) motor score.
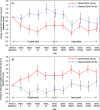
Results: All 41 parent study participants were enrolled. The primary outcome decreased by 26.7±20.7% in patients on GDNF for 80 weeks (GDNF/GDNF; N = 21) and 27.6±23.6% in patients on placebo for 40 weeks followed by GDNF for 40 weeks (placebo/GDNF, N = 20; least squares mean difference: 0.4%, 95% CI: -13.9, 14.6, p = 0.96). Secondary endpoints did not show significant differences between the groups at Week 80 either. Prespecified comparisons between GDNF/GDNF at Week 80 and placebo/GDNF at Week 40 showed significant differences for mean OFF state UPDRS motor (-9.6±6.7 vs. -3.8±4.2 points, p = 0.0108) and activities of daily living score (-6.9±5.5 vs. -1.0±3.7 points, p = 0.0003). No treatment-emergent safety concerns were identified.
Conclusions: The aggregate study results, from the parent and open-label extension suggest that future testing with GDNF will likely require an 80- rather than a 40-week randomized treatment period and/or a higher dose.
Keywords: Glial cell line-derived neurotrophic factor; Parkinson’s disease; convection enhanced delivery; neurorestoration.
Conflict of interest statement
Matthias Luz, Lara Longpre, Chris Boiko, Greg A. Johnson, H. Christian Fibiger and Erich Mohr are employed by and have shares and/or share options with MedGenesis Therapeutix Inc., owners of the license for GDNF. Lynn Barclay is a consultant to MedGenesis Therapeutix, Inc. Max Woolley, Rob Harrison, Owen Lewis, Gemma Pritchard, Mike Howell, Charlie Irving, David Johnson, Suk Kinch and Paul Skinner are employees of Renishaw plc, contract manufactures of the drug-delivery system. Steven Gill is the Medical Director of Renishaw plc and is the inventor of the drug delivery system from which he may have a future royalty share. He is on the scientific advisory board of MedGenesis Therapeutix Inc. for which he is reimbursed with share options. No other potential conflict of interest relevant to this article was reported.
Figures

References
-
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138, 155–175. - PubMed
-
- Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9, 589–595. - PubMed
-
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B (2005) Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg 102, 216–222. - PubMed
-
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59, 459–466. - PubMed
-
- Whone AL, Luz M, Boca M, Woolley M, Mooney L, Dharia S, Broadfoot J, Cronin S, Schroers S, Barua N, Longpre L, Barclay CL, Boiko C, Johnson G, Fibiger C, Harrison R, Lewis O, Pritchard G, Howell M, Irving C, Johnson D, Kinch S, Marshall C, Lawrence A, Blinder S, Sossi V, Stoessl AJ, Skinner P, Mohr E, Gill SS (2019) Randomised trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 142, 512–525. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

