Best Practices for Obtaining Genomic Consent in Pediatric Traumatic Brain Injury Research
- PMID: 30829926
- PMCID: PMC6400301
- DOI: 10.1097/NNR.0000000000000335
Best Practices for Obtaining Genomic Consent in Pediatric Traumatic Brain Injury Research
Abstract
Background: Precision health relies on large sample sizes to ensure adequate power, generalizability, and replicability; however, a critical first step to any study is the successful recruitment of participants.
Objectives: This study seeks to explore how the enrollment strategies used in a parent study contributed to the high consent rates, establish current best practices that can be used in future studies, and identify additional factors that contribute to consent into pediatric traumatic brain injury biobanks.
Methods: Retrospective secondary analysis of data from a parent study with high consent rates was examined to explore factors affecting consent into biobanking studies.
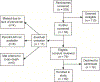
Results: Of the 76 subjects who were approached, met the eligibility criteria, and reviewed the consent form, only 16 (21.1%) declined to participate. The consented group (n = 60) represents 64.5% of those who met the eligibility criteria upon initial screening (n = 93) and 78.9% of those with confirmed eligibility (n = 76). Analysis of screening data suggested there were no major barriers to consenting individuals into this pediatric traumatic brain injury biobank.
Discussion: There were no demographic or research-related characteristics that significantly explained enrollment. Ethically, to obtain true informed consent, parents need to understand only their child's diagnosis, prognosis, and medical care, as well as the purpose of the proposed research and its risks and benefits. Researchers need to implement best practices, including a comprehensive review of census data to identify eligible participants to approach, a prescreening protocol, and effective consenting process to obtain informed consent so that precision care initiatives can be pursued.
Conflict of interest statement
Figures

Similar articles
-
Best practices for recruitment of adolescents for biobanking and precision health research: a retrospective analysis comparing juvenile idiopathic arthritis cases with healthy controls.Pediatr Rheumatol Online J. 2021 Dec 4;19(1):169. doi: 10.1186/s12969-021-00652-9. Pediatr Rheumatol Online J. 2021. PMID: 34863185 Free PMC article.
-
Consenting to pediatric critical care research: understanding the perspective of parents.Dynamics. 2013 Fall;24(3):18-24. Dynamics. 2013. PMID: 24288878
-
Opinions of Adolescents and Parents About Pediatric Biobanking.J Adolesc Health. 2016 Apr;58(4):474-480. doi: 10.1016/j.jadohealth.2015.12.015. J Adolesc Health. 2016. PMID: 27013273
-
Informed consent/assent in children. Statement of the Ethics Working Group of the Confederation of European Specialists in Paediatrics (CESP).Eur J Pediatr. 2003 Sep;162(9):629-33. doi: 10.1007/s00431-003-1193-z. Epub 2003 Jul 19. Eur J Pediatr. 2003. PMID: 12884032 Review.
-
Informed consent in pediatric research.Paediatr Drugs. 2015 Feb;17(1):5-11. doi: 10.1007/s40272-014-0108-y. Paediatr Drugs. 2015. PMID: 25420675 Review.
Cited by
-
Best practices for recruitment of adolescents for biobanking and precision health research: a retrospective analysis comparing juvenile idiopathic arthritis cases with healthy controls.Pediatr Rheumatol Online J. 2021 Dec 4;19(1):169. doi: 10.1186/s12969-021-00652-9. Pediatr Rheumatol Online J. 2021. PMID: 34863185 Free PMC article.
-
A Novel Clinical Research Modality for Enrolling Diverse Participants Using a Diverse Team.Brain Sci. 2020 Jul 8;10(7):434. doi: 10.3390/brainsci10070434. Brain Sci. 2020. PMID: 32650502 Free PMC article.
-
Recruitment principles and strategies for supportive care research in pediatric oncology.BMC Med Res Methodol. 2021 Aug 28;21(1):178. doi: 10.1186/s12874-021-01371-1. BMC Med Res Methodol. 2021. PMID: 34454413 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

