Antibody-suppressor CD8+ T Cells Require CXCR5
- PMID: 30830040
- PMCID: PMC6713619
- DOI: 10.1097/TP.0000000000002683
Antibody-suppressor CD8+ T Cells Require CXCR5
Abstract
Background: We previously reported the novel activity of alloprimed CD8 T cells that suppress posttransplant alloantibody production. The purpose of the study is to investigate the expression and role of CXCR5 on antibody-suppressor CD8 T-cell function.
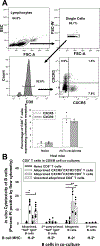
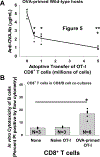
Methods: C57BL/6 mice were transplanted with FVB/N hepatocytes. Alloprimed CD8 T cells were retrieved on day 7 from hepatocyte transplant recipients. Unsorted or flow-sorted (CXCR5CXCR3 and CXCR3CXCR5) alloprimed CD8 T-cell subsets were analyzed for in vitro cytotoxicity and capacity to inhibit in vivo alloantibody production following adoptive transfer into C57BL/6 or high alloantibody-producing CD8 knock out (KO) hepatocyte transplant recipients. Alloantibody titer was assessed in CD8 KO mice reconstituted with naive CD8 T cells retrieved from C57BL/6, CXCR5 KO, or CXCR3 KO mice. Antibody suppression by ovalbumin (OVA)-primed monoclonal OVA-specific t-cell receptor transgenic CD8+ T cells (OT-I) CXCR5 or CXCR3 CD8 T-cell subsets was also investigated.
Results: Alloprimed CXCR5CXCR3CD8 T cells mediated in vitro cytotoxicity of alloprimed "self" B cells, while CXCR3CXCR5CD8 T cells did not. Only flow-sorted alloprimed CXCR5CXCR3CD8 T cells (not flow-sorted alloprimed CXCR3CXCR5CD8 T cells) suppressed alloantibody production and enhanced graft survival when transferred into transplant recipients. Unlike CD8 T cells from wild-type or CXCR3 KO mice, CD8 T cells from CXCR5 KO mice do not develop alloantibody-suppressor function. Similarly, only flow-sorted CXCR5CXCR3 (and not CXCR3CXCR5) OVA-primed OT-I CD8 T cells mediated in vivo suppression of anti-OVA antibody production.
Conclusions: These data support the conclusion that expression of CXCR5 by antigen-primed CD8 T cells is critical for the function of antibody-suppressor CD8 T cells.
Conflict of interest statement
Figures




Comment in
-
Fate of CD8+: Cytotoxic or Suppressor T Cells in Antibody-mediated Rejection in Solid Organ Transplantation?Transplantation. 2019 Sep;103(9):1756-1757. doi: 10.1097/TP.0000000000002684. Transplantation. 2019. PMID: 30817404 No abstract available.
References
-
- Brooks AM, Carter V, Liew A, et al. De Novo Donor-Specific HLA Antibodies Are Associated With Rapid Loss of Graft Function Following Islet Transplantation in Type 1 Diabetes. Am J Transplant. 2015;15(12):3239–3246. - PubMed
-
- Blanchard D, Gaillard C, Hermann P, et al. Role of CD40 antigen and interleukin-2 in T cell-dependent human B lymphocyte growth. Eur J Immunol. 1994;24(2):330–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

