Analysis of Morbidity and Outcomes Associated With Use of Subdural Grids vs Stereoelectroencephalography in Patients With Intractable Epilepsy
- PMID: 30830149
- PMCID: PMC6563575
- DOI: 10.1001/jamaneurol.2019.0098
Analysis of Morbidity and Outcomes Associated With Use of Subdural Grids vs Stereoelectroencephalography in Patients With Intractable Epilepsy
Abstract
Importance: A major change has occurred in the evaluation of epilepsy with the availability of robotic stereoelectroencephalography (SEEG) for seizure localization. However, the comparative morbidity and outcomes of this minimally invasive procedure relative to traditional subdural electrode (SDE) implantation are unknown.
Objective: To perform a comparative analysis of the relative efficacy, procedural morbidity, and epilepsy outcomes consequent to SEEG and SDE in similar patient populations and performed by a single surgeon at 1 center.
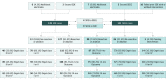
Design, setting and participants: Overall, 239 patients with medically intractable epilepsy underwent 260 consecutive intracranial electroencephalographic procedures to localize their epilepsy. Procedures were performed from November 1, 2004, through June 30, 2017, and data were analyzed in June 2017 and August 2018.
Interventions: Implantation of SDE using standard techniques vs SEEG using a stereotactic robot, followed by resection or laser ablation of the seizure focus.
Main outcomes and measures: Length of surgical procedure, surgical complications, opiate use, and seizure outcomes using the Engel Epilepsy Surgery Outcome Scale.
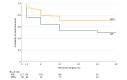
Results: Of the 260 cases included in the study (54.6% female; mean [SD] age at evaluation, 30.3 [13.1] years), the SEEG (n = 121) and SDE (n = 139) groups were similar in age (mean [SD], 30.1 [12.2] vs 30.6 [13.8] years), sex (47.1% vs 43.9% male), numbers of failed anticonvulsants (mean [SD], 5.7 [2.5] vs 5.6 [2.5]), and duration of epilepsy (mean [SD], 16.4 [12.0] vs17.2 [12.1] years). A much greater proportion of SDE vs SEEG cases were lesional (99 [71.2%] vs 53 [43.8%]; P < .001). Seven symptomatic hemorrhagic sequelae (1 with permanent neurological deficit) and 3 infections occurred in the SDE cohort with no clinically relevant complications in the SEEG cohort, a marked difference in complication rates (P = .003). A greater proportion of SDE cases resulted in resection or ablation compared with SEEG cases (127 [91.4%] vs 90 [74.4%]; P < .001). Favorable epilepsy outcomes (Engel class I [free of disabling seizures] or II [rare disabling seizures]) were observed in 57 of 75 SEEG cases (76.0%) and 59 of 108 SDE cases (54.6%; P = .003) amongst patients undergoing resection or ablation, at 1 year. An analysis of only nonlesional cases revealed good outcomes in 27 of 39 cases (69.2%) vs 9 of 26 cases (34.6%) at 12 months in SEEG and SDE cohorts, respectively (P = .006). When considering all patients undergoing evaluation, not just those undergoing definitive procedures, favorable outcomes (Engel class I or II) for SEEG compared with SDE were similar (57 of 121 [47.1%] vs 59 of 139 [42.4%] at 1 year; P = .45).
Conclusions and relevance: This direct comparison of large matched cohorts undergoing SEEG and SDE implantation reveals distinctly better procedural morbidity favoring SEEG. These modalities intrinsically evaluate somewhat different populations, with SEEG being more versatile and applicable to a range of scenarios, including nonlesional and bilateral cases, than SDE. The significantly favorable adverse effect profile of SEEG should factor into decision making when patients with pharmacoresistant epilepsy are considered for intracranial evaluations.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

