From neural crest cells to melanocytes: cellular plasticity during development and beyond
- PMID: 30830237
- PMCID: PMC11105195
- DOI: 10.1007/s00018-019-03049-w
From neural crest cells to melanocytes: cellular plasticity during development and beyond
Abstract
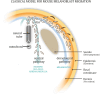
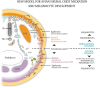
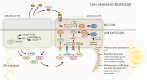
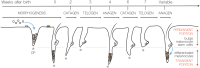
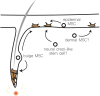
Here, we review melanocyte development and how the embryonic melanoblast, although specified to become a melanocyte, is prone to cellular plasticity and is not fully committed to the melanocyte lineage. Even fully differentiated and pigment-producing melanocytes do not always have a stable phenotype. The gradual lineage restriction of neural crest cells toward the melanocyte lineage is determined by both cell-intrinsic and extracellular signals in which differentiation and pathfinding ability reciprocally influence each other. These signals are leveraged by subtle differences in timing and axial positioning. The most extensively studied migration route is the dorsolateral path between the dermomyotome and the prospective epidermis, restricted to melanoblasts. In addition, the embryonic origin of the skin dermis through which neural crest derivatives migrate may also affect the segregation between melanogenic and neurogenic cells in embryos. It is widely accepted that, irrespective of the model organism studied, the immediate precursor of both melanoblast and neurogenic populations is a glial-melanogenic bipotent progenitor. Upon exposure to different conditions, melanoblasts may differentiate into other neural crest-derived lineages such as neuronal cells and vice versa. Key factors that regulate melanoblast migration and patterning will regulate melanocyte homeostasis during different stages of hair cycling in postnatal hair follicles.
Keywords: Cellular plasticity; EMT; Melanocytes; Migration; Neural crest; ZEB proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
[Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells].Biol Aujourdhui. 2011;205(1):53-61. doi: 10.1051/jbio/2011008. Epub 2011 Apr 19. Biol Aujourdhui. 2011. PMID: 21501576 Review. French.
-
The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube.Dev Biol. 1998 Aug 15;200(2):234-46. doi: 10.1006/dbio.1998.8963. Dev Biol. 1998. PMID: 9705230
-
Hyperpigmentation in the Silkie fowl correlates with abnormal migration of fate-restricted melanoblasts and loss of environmental barrier molecules.Dev Dyn. 2001 Mar;220(3):212-25. doi: 10.1002/1097-0177(20010301)220:3<212::AID-DVDY1105>3.0.CO;2-9. Dev Dyn. 2001. PMID: 11241830
-
The roles of Frizzled-3 and Wnt3a on melanocyte development: in vitro studies on neural crest cells and melanocyte precursor cell lines.J Dermatol Sci. 2014 Aug;75(2):100-8. doi: 10.1016/j.jdermsci.2014.04.012. Epub 2014 May 5. J Dermatol Sci. 2014. PMID: 24815018
-
Modeling melanoblast development.Cell Mol Life Sci. 2013 Mar;70(6):1067-79. doi: 10.1007/s00018-012-1112-4. Epub 2012 Aug 23. Cell Mol Life Sci. 2013. PMID: 22915137 Free PMC article. Review.
Cited by
-
Single cell transcriptional zonation of human psoriasis skin identifies an alternative immunoregulatory axis conducted by skin resident cells.Cell Death Dis. 2021 May 6;12(5):450. doi: 10.1038/s41419-021-03724-6. Cell Death Dis. 2021. PMID: 33958582 Free PMC article.
-
Auditory and speech outcomes of cochlear implantation in patients with Waardenburg syndrome: a meta-analysis.Front Neurol. 2024 Jul 11;15:1372736. doi: 10.3389/fneur.2024.1372736. eCollection 2024. Front Neurol. 2024. PMID: 39055322 Free PMC article.
-
Melanoblast transcriptome analysis reveals pathways promoting melanoma metastasis.Nat Commun. 2020 Jan 16;11(1):333. doi: 10.1038/s41467-019-14085-2. Nat Commun. 2020. PMID: 31949145 Free PMC article.
-
Why Does the Face Predict the Brain? Neural Crest Induction, Craniofacial Morphogenesis, and Neural Circuit Development.Front Physiol. 2020 Dec 11;11:610970. doi: 10.3389/fphys.2020.610970. eCollection 2020. Front Physiol. 2020. PMID: 33362582 Free PMC article. Review.
-
Dynamic changes in the skin transcriptome for the melanin pigmentation in embryonic chickens.Poult Sci. 2025 Jan;104(1):104210. doi: 10.1016/j.psj.2024.104210. Epub 2024 Aug 13. Poult Sci. 2025. PMID: 39693959 Free PMC article.
References
-
- Le Douarin NM, Kalcheim C. The neural crest. Cambridge: Cambridge University Press; 1999.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

