Absolute numbers of regulatory T cells and neutrophils in corticosteroid-free patients are predictive for response to bevacizumab in recurrent glioblastoma patients
- PMID: 30830269
- PMCID: PMC6529384
- DOI: 10.1007/s00262-019-02317-9
Absolute numbers of regulatory T cells and neutrophils in corticosteroid-free patients are predictive for response to bevacizumab in recurrent glioblastoma patients
Abstract
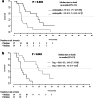
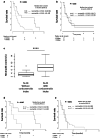
Bevacizumab (Bv) remains frequently prescribed in glioblastoma (GBM) patients, especially at recurrence. We conducted a prospective clinical trial with 29 recurrent GBM patients treated with Bv alone with a longitudinal follow-up of different circulating immune cells [complete blood count, myeloid-derived suppressor cells (MDSCs), classical, intermediate, non-classical and Tie2 monocytes, VEGFR1+ and regulatory T cells (Treg)]. We observed a significant increase for leucocytes, neutrophils, eosinophils and classical monocytes and a decrease for the fraction of Treg during the treatment. The best prognostic values for survival under Bv were obtained for basal neutrophils and Treg. Counts below 3.9 G/L for neutrophils and above 0.011 G/L for Treg were associated with an overall survival of 17.5 and 19.9 months, respectively, as compared with 5.4 and 5.6 months, respectively, for counts above and below these cutoffs (p = 0.004 and p < 0.001). No prognostic impact was observed for neutrophils in a retrospective cohort of 26 patients treated with nitrosoureas alone. In another retrospective validation cohort of 61 GBM patients treated at recurrence with a Bv-containing regimen, an interaction was observed between neutrophils and corticosteroid intake. The predictive value of neutrophils on survival under Bv was lost in patients treated with corticosteroids, when steroid-free patients with a low neutrophil count had a particularly long median survival of 3.4 years. These two simply accessible criteria (basal neutrophils and steroid intake) could be used to reserve this relatively costly treatment for patients likely to be the most responsive to Bv and prevent unnecessary side effects in others.
Keywords: Bevacizumab; Biomarker; Glioblastoma; Neutrophils; Regulatory T cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Long-term survival in patients with recurrent glioblastoma treated with bevacizumab: a multicentric retrospective study.J Neurooncol. 2019 Sep;144(2):419-426. doi: 10.1007/s11060-019-03245-5. Epub 2019 Jul 19. J Neurooncol. 2019. PMID: 31325146
-
Phase II study of ERC1671 plus bevacizumab versus bevacizumab plus placebo in recurrent glioblastoma: interim results and correlations with CD4+ T-lymphocyte counts.CNS Oncol. 2018 Jul 1;7(3):CNS22. doi: 10.2217/cns-2018-0009. Epub 2018 Aug 29. CNS Oncol. 2018. PMID: 30157683 Free PMC article. Clinical Trial.
-
Cerebral blood volume and apparent diffusion coefficient - Valuable predictors of non-response to bevacizumab treatment in patients with recurrent glioblastoma.J Neurol Sci. 2019 Oct 15;405:116433. doi: 10.1016/j.jns.2019.116433. Epub 2019 Aug 23. J Neurol Sci. 2019. PMID: 31476621
-
The role of bevacizumab in the treatment of glioblastoma.J Neurooncol. 2017 Jul;133(3):455-467. doi: 10.1007/s11060-017-2477-x. Epub 2017 May 19. J Neurooncol. 2017. PMID: 28527008 Review.
-
Use of bevacizumab in recurrent glioblastoma.CNS Oncol. 2015;4(3):157-69. doi: 10.2217/cns.15.8. Epub 2015 Apr 23. CNS Oncol. 2015. PMID: 25906439 Free PMC article. Review.
Cited by
-
Immune cell landscape and immunotherapy of medulloblastoma.Pediatr Investig. 2021 Jun 21;5(4):299-309. doi: 10.1002/ped4.12261. eCollection 2021 Dec. Pediatr Investig. 2021. PMID: 34938973 Free PMC article. Review.
-
Research Supporting a Pilot Study of Metronomic Dapsone during Glioblastoma Chemoirradiation.Med Sci (Basel). 2021 Feb 16;9(1):12. doi: 10.3390/medsci9010012. Med Sci (Basel). 2021. PMID: 33669324 Free PMC article.
-
Immunotherapy for Recurrent Glioma-From Bench to Bedside.Cancers (Basel). 2023 Jun 30;15(13):3421. doi: 10.3390/cancers15133421. Cancers (Basel). 2023. PMID: 37444531 Free PMC article. Review.
-
Effect of radiochemotherapy on peripheral immune response in glioblastoma.Cancer Immunol Immunother. 2024 May 16;73(7):133. doi: 10.1007/s00262-024-03722-5. Cancer Immunol Immunother. 2024. PMID: 38753169 Free PMC article.
-
Update on Chemotherapeutic Approaches and Management of Bevacizumab Usage for Glioblastoma.Pharmaceuticals (Basel). 2020 Dec 16;13(12):470. doi: 10.3390/ph13120470. Pharmaceuticals (Basel). 2020. PMID: 33339404 Free PMC article. Review.
References
-
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. - DOI - PubMed
-
- Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. - DOI - PMC - PubMed
-
- Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–953. doi: 10.1016/S1470-2045(14)70314-6. - DOI - PubMed
-
- Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Le Rhun E, Dubois F, Weller M, von Deimling A, Golfinopoulos V, Bromberg JC, Platten M, Klein M, van den Bent MJ. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377:1954–1963. doi: 10.1056/NEJMoa1707358. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

