NIST lipidomics workflow questionnaire: an assessment of community-wide methodologies and perspectives
- PMID: 30830346
- PMCID: PMC11493135
- DOI: 10.1007/s11306-018-1340-1
NIST lipidomics workflow questionnaire: an assessment of community-wide methodologies and perspectives
Abstract
Introduction: Efforts to harmonize lipidomic methodologies have been limited within the community. Here, we aimed to capitalize on the recent National Institute of Standards and Technology lipidomics interlaboratory comparison exercise by implementing a questionnaire that assessed current methodologies, quantitation strategies, standard operating procedures (SOPs), and quality control activities employed by the lipidomics community.
Objectives: Lipidomics is a rapidly developing field with diverse applications. At present, there are no community-vetted methods to assess measurement comparability or data quality. Thus, a major impetus of this questionnaire was to profile current efforts, highlight areas of need, and establish future objectives in an effort to harmonize lipidomics workflows.
Methods: The 54-question survey inquired about laboratory demographics, lipidomic methodologies and SOPs, analytical platforms, quantitation, reference materials, quality control procedures, and opinions regarding challenges existing within the community.
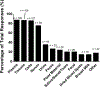
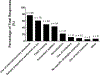
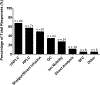
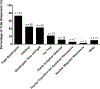
Results: A total of 125 laboratories participated in the questionnaire. A broad overview of results highlighted a wide methodological diversity within current lipidomic workflows. The impact of this diversity on lipid measurement and quantitation is currently unknown and needs to be explored further. While some laboratories do incorporate SOPs and quality control activities, these concepts have not been fully embraced by the community. The top five perceived challenges within the lipidomics community were a lack of standardization amongst methods/protocols, lack of lipid standards, software/data handling and quantification, and over-reporting/false positives.
Conclusion: The questionnaire provided an overview of current lipidomics methodologies and further promoted the need for community-accepted guidelines and protocols. The questionnaire also served as a platform to help determine and prioritize metrological issues to be investigated.
Keywords: Harmonization; Lipidomics; Methodology; Quality Control; Quantitation; Questionnaire.
Conflict of interest statement
Compliance with ethical standards
Figures



References
-
- Bowden JA, Heckert A, Ulmer CZ, Jones CM, Koelmel JP, Abdullah L, et al. (2017a). Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using standard reference material 1950 metabolites in frozen human plasma. Journal of Lipid Research. 10.1194/jlr.M079012. - DOI - PMC - PubMed
-
- Bowden JA, Ulmer CZ, Jones CM, & Heckert NA (2017b). Lipid concentrations in standard reference material (SRM) 1950: Results from an interlaboratory comparison exercise for lipidomics. National Institute of Standards and Technology (NIST) IR 8185. http://nvlpubs.nist.gov/nistpubs/ir/2017/NIST.IR.8185.pdf.
-
- Dunn WB, Broadhurst DI, Edison A, Guillou C, Viant MR, Bearden DW & Beger RD (2017). Quality assurance and quality control processes: Summary of a metabolomics community questionnaire. Metabolomics, 13, 50.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
