Unexpected differential metabolic responses of Campylobacter jejuni to the abundant presence of glutamate and fucose
- PMID: 30830405
- PMCID: PMC6208705
- DOI: 10.1007/s11306-018-1438-5
Unexpected differential metabolic responses of Campylobacter jejuni to the abundant presence of glutamate and fucose
Abstract
Introduction: Campylobacter jejuni is the leading cause of foodborne bacterial enteritis in humans, and yet little is known in regard to how genetic diversity and metabolic capabilities among isolates affect their metabolic phenotype and pathogenicity.
Objectives: For instance, the C. jejuni 11168 strain can utilize both L-fucose and L-glutamate as a carbon source, which provides the strain with a competitive advantage in some environments and in this study we set out to assess the metabolic response of C. jejuni 11168 to the presence of L-fucose and L-glutamate in the growth medium.
Methods: To achieve this, untargeted hydrophilic liquid chromatography coupled to mass spectrometry was used to obtain metabolite profiles of supernatant extracts obtained at three different time points up to 24 h.
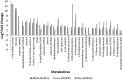
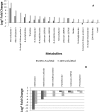
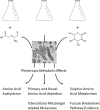
Results: This study identified both the depletion and the production and subsequent release of a multitude of expected and unexpected metabolites during the growth of C. jejuni 11168 under three different conditions. A large set of standards allowed identification of a number of metabolites. Further mass spectrometry fragmentation analysis allowed the additional annotation of substrate-specific metabolites. The results show that C. jejuni 11168 upon L-fucose addition indeed produces degradation products of the fucose pathway. Furthermore, methionine was faster depleted from the medium, consistent with previously-observed methionine auxotrophy.
Conclusions: Moreover, a multitude of not previously annotated metabolites in C. jejuni were found to be increased specifically upon L-fucose addition. These metabolites may well play a role in the pathogenicity of this C. jejuni strain.
Keywords: Campylobacter jejuni; HILIC chromatography; Mass spectrometry fragmentation; Metabolomics; Sulphur metabolism.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors and all materials used were from in vitro cultures of bacteria.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures


Similar articles
-
L-fucose utilization provides Campylobacter jejuni with a competitive advantage.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):7194-9. doi: 10.1073/pnas.1014125108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482772 Free PMC article.
-
Phenotypic and genotypic evidence for L-fucose utilization by Campylobacter jejuni.J Bacteriol. 2011 Mar;193(5):1065-75. doi: 10.1128/JB.01252-10. Epub 2010 Dec 30. J Bacteriol. 2011. PMID: 21193610 Free PMC article.
-
Metabolomic profiling of Campylobacter jejuni with resistance gene ermB by ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry and tandem quadrupole mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Mar 15;1079:62-68. doi: 10.1016/j.jchromb.2018.02.009. Epub 2018 Feb 10. J Chromatogr B Analyt Technol Biomed Life Sci. 2018. PMID: 29453015
-
Nutrient acquisition and metabolism by Campylobacter jejuni.Front Cell Infect Microbiol. 2012 Feb 7;2:5. doi: 10.3389/fcimb.2012.00005. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919597 Free PMC article. Review.
-
Defining the metabolic requirements for the growth and colonization capacity of Campylobacter jejuni.Front Cell Infect Microbiol. 2014 Sep 29;4:137. doi: 10.3389/fcimb.2014.00137. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25325018 Free PMC article. Review.
Cited by
-
Revisiting Campylobacter jejuni Virulence and Fitness Factors: Role in Sensing, Adapting, and Competing.Front Cell Infect Microbiol. 2021 Feb 3;10:607704. doi: 10.3389/fcimb.2020.607704. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33614526 Free PMC article. Review.
-
The Role of luxS in Campylobacter jejuni Beyond Intercellular Signaling.Microbiol Spectr. 2023 Feb 1;11(2):e0257222. doi: 10.1128/spectrum.02572-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36722966 Free PMC article.
-
Comparative Analysis of L-Fucose Utilization and Its Impact on Growth and Survival of Campylobacter Isolates.Front Microbiol. 2022 Apr 29;13:872207. doi: 10.3389/fmicb.2022.872207. eCollection 2022. Front Microbiol. 2022. PMID: 35572645 Free PMC article.
-
The gastrointestinal pathogen Campylobacter jejuni metabolizes sugars with potential help from commensal Bacteroides vulgatus.Commun Biol. 2020 Jan 7;3(1):2. doi: 10.1038/s42003-019-0727-5. Commun Biol. 2020. PMID: 31925306 Free PMC article.
-
Inhibition of Campylobacter jejuni Biofilm Formation by D-Amino Acids.Antibiotics (Basel). 2020 Nov 23;9(11):836. doi: 10.3390/antibiotics9110836. Antibiotics (Basel). 2020. PMID: 33238583 Free PMC article.
References
-
- Alghafari, W. T. (2016). Metabolic Diversity in Campylobacter jejuni. Ph.D. thesis, Heriot-Watt University.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
