Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS Asia
- PMID: 30830724
- PMCID: PMC7379575
- DOI: 10.1111/dom.13681
Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS Asia
Abstract
Aim: Phase III, randomized, double-blind study evaluating the efficacy and safety of ertugliflozin in Asian patients with type 2 diabetes mellitus (T2DM) inadequately controlled on metformin, including evaluation in the China subpopulation.
Materials and methods: A 26-week, double-blind study of 506 Asian patients (80.2% from mainland China), randomized 1:1:1 to placebo, ertugliflozin 5- or 15 mg, was performed. Primary endpoint was change from baseline in HbA1c at week 26. Secondary endpoints were change from baseline at week 26 in fasting plasma glucose (FPG), body weight (BW), systolic/diastolic blood pressure (SBP/DBP), and proportion of patients with HbA1c <7.0%. Hypotheses for the primary endpoint and FPG and BW secondary endpoints were tested in the China subpopulation.
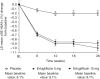
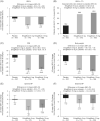
Results: At week 26, least squares mean (95% CI) change from baseline HbA1c was significantly greater with ertugliflozin 5- and 15 mg versus placebo: -1.0% (-1.1, -0.9), -0.9% (-1.0, -0.8), -0.2% (-0.3, -0.1), respectively. Ertugliflozin significantly reduced FPG, BW and SBP. Reductions in DBP with ertugliflozin were not significant. At week 26, 16.2%, 38.2% and 40.8% of patients had HbA1c <7.0% with placebo, ertugliflozin 5- and 15 mg, respectively. 59.3%, 56.5% and 53.3% of patients experienced adverse events with placebo, ertugliflozin 5- and 15 mg, respectively. Incidence of symptomatic hypoglycaemia was higher for ertugliflozin 15 mg vs placebo. Results in the China subpopulation were consistent.
Conclusions: Ertugliflozin significantly improved glycaemic control and reduced BW and SBP in Asian patients with T2DM. Ertugliflozin was generally well-tolerated. Results in the China subpopulation were consistent with the overall population. ClinicalTrials.gov: NCT02630706.
Keywords: Asia; SGLT2 inhibitor; ertugliflozin; type 2 diabetes mellitus.
© 2019 John Wiley & Sons Ltd.
Conflict of interest statement
Author contributions
All authors critically reviewed the draft manuscript and approved the final version of the manuscript for publication. M.Y., W.W., Y.M., S.P., P.Y. and S.G.T. were involved in the conception/design of the study. L.J., Y.L., H.M. and Y.X. were involved in the acquisition of data for the study. All authors were involved in data analysis and interpretation of the data.
Figures
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017.
-
- Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948‐959. - PubMed
-
- Li H, Oldenburg B, Chamberlain C, et al. Diabetes prevalence and determinants in adults in China mainland from 2000 to 2010: a systematic review. Diabetes Res Clin Pract. 2012;98:226‐235. - PubMed
-
- Fitchett D, Inzucchi SE, Lachin JM, et al. Cardiovascular mortality reduction with empagliflozin in patients with type 2 diabetes and cardiovascular disease. J Am Coll Cardiol. 2018;71:364‐367. - PubMed
-
- El Masri D, Ghosh S, Jaber LA. Safety and efficacy of sodium‐glucose cotransporter 2 (SGLT2) inhibitors in type 1 diabetes: a systematic review and meta‐analysis. Diabetes Res Clin Pract. 2018;137:83‐92. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

