Vaccine induction of antibodies and tissue-resident CD8+ T cells enhances protection against mucosal SHIV-infection in young macaques
- PMID: 30830870
- PMCID: PMC6478416
- DOI: 10.1172/jci.insight.126047
Vaccine induction of antibodies and tissue-resident CD8+ T cells enhances protection against mucosal SHIV-infection in young macaques
Abstract
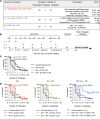
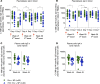
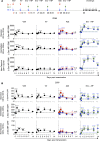
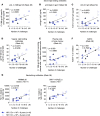
Antibodies and cytotoxic T cells represent 2 arms of host defense against pathogens. We hypothesized that vaccines that induce both high-magnitude CD8+ T cell responses and antibody responses might confer enhanced protection against HIV. To test this hypothesis, we immunized 3 groups of nonhuman primates: (a) Group 1, which includes sequential immunization regimen involving heterologous viral vectors (HVVs) comprising vesicular stomatitis virus, vaccinia virus, and adenovirus serotype 5-expressing SIVmac239 Gag; (b) Group 2, which includes immunization with a clade C HIV-1 envelope (Env) gp140 protein adjuvanted with nanoparticles containing a TLR7/8 agonist (3M-052); and (c) Group 3, which includes a combination of both regimens. Immunization with HVVs induced very high-magnitude Gag-specific CD8+ T cell responses in blood and tissue-resident CD8+ memory T cells in vaginal mucosa. Immunization with 3M-052 adjuvanted Env protein induced robust and persistent antibody responses and long-lasting innate responses. Despite similar antibody titers in Groups 2 and 3, there was enhanced protection in the younger animals in Group 3, against intravaginal infection with a heterologous SHIV strain. This protection correlated with the magnitude of the serum and vaginal Env-specific antibody titers on the day of challenge. Thus, vaccination strategies that induce both CD8+ T cell and antibody responses can confer enhanced protection against infection.
Keywords: AIDS vaccine; AIDS/HIV; Adaptive immunity; Vaccines.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

