The BAG3-dependent and -independent roles of cardiac small heat shock proteins
- PMID: 30830872
- PMCID: PMC6478417
- DOI: 10.1172/jci.insight.126464
The BAG3-dependent and -independent roles of cardiac small heat shock proteins
Abstract
Small heat shock proteins (sHSPs) comprise an important protein family that is ubiquitously expressed, is highly conserved among species, and has emerged as a critical regulator of protein folding. While these proteins are functionally important for a variety of tissues, an emerging field of cardiovascular research reveals sHSPs are also extremely important for maintaining normal cardiac function and regulating the cardiac stress response. Notably, numerous mutations in genes encoding sHSPs have been associated with multiple cardiac diseases. sHSPs (HSPB5, HSPB6, and HSPB8) have been described as mediating chaperone functions within the heart by interacting with the cochaperone protein BCL-2-associated anthanogene 3 (BAG3); however, recent reports indicate that sHSPs (HSPB7) can perform other BAG3-independent functions. Here, we summarize the cardiac functions of sHSPs and present the notion that cardiac sHSPs function via BAG3-dependent or -independent pathways.
Conflict of interest statement
Figures
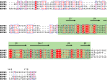
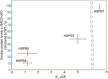
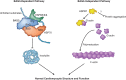
References
-
- Vos MJ, Kanon B, Kampinga HH. HSPB7 is a SC35 speckle resident small heat shock protein. Biochim Biophys Acta. 2009;1793(8):1343–1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

