Cleavage factor 25 deregulation contributes to pulmonary fibrosis through alternative polyadenylation
- PMID: 30830875
- PMCID: PMC6486348
- DOI: 10.1172/JCI122106
Cleavage factor 25 deregulation contributes to pulmonary fibrosis through alternative polyadenylation
Abstract
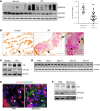
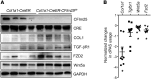
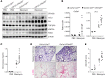
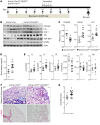
Idiopathic pulmonary fibrosis (IPF) is a chronic and deadly disease with a poor prognosis and few treatment options. Pathological remodeling of the extracellular matrix (ECM) by myofibroblasts is a key factor that drives disease pathogenesis, although the underlying mechanisms remain unknown. Alternative polyadenylation (APA) has recently been shown to play a major role in cellular responses to stress by driving the expression of fibrotic factors and ECMs through altering microRNA sensitivity, but a connection to IPF has not been established. Here, we demonstrate that CFIm25, a global regulator of APA, is down-regulated in the lungs of patients with IPF and mice with pulmonary fibrosis, with its expression selectively reduced in alpha-smooth muscle actin (α-SMA) positive fibroblasts. Following the knockdown of CFIm25 in normal human lung fibroblasts, we identified 808 genes with shortened 3'UTRs, including those involved in the transforming growth factor-β signaling pathway, the Wnt signaling pathway, and cancer pathways. The expression of key pro-fibrotic factors can be suppressed by CFIm25 overexpression in IPF fibroblasts. Finally, we demonstrate that deletion of CFIm25 in fibroblasts or myofibroblast precursors using either the Col1a1 or the Foxd1 promoter enhances pulmonary fibrosis after bleomycin exposure in mice. Taken together, our results identified CFIm25 down-regulation as a novel mechanism to elevate pro-fibrotic gene expression in pulmonary fibrosis.
Keywords: Fibrosis; Pulmonology.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

