The role of FOXP3+ regulatory T cells in human autoimmune and inflammatory diseases
- PMID: 30830965
- PMCID: PMC6591146
- DOI: 10.1111/cei.13288
The role of FOXP3+ regulatory T cells in human autoimmune and inflammatory diseases
Abstract
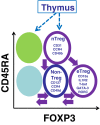
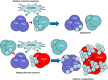
CD4+ regulatory T cells (Treg ) expressing the forkhead box protein 3 (FOXP3) transcription factor (Tregs ) are instrumental for the prevention of autoimmune diseases. There is increasing evidence that the human T regulatory population is highly heterogeneous in phenotype and function. Numerous studies conducted in human autoimmune diseases have shown that Treg cells are impaired either in their suppressive function, in number, or both. However, the contribution of the FOXP3+ Treg subpopulations to the development of autoimmunity has not been delineated in detail. Rare genetic disorders that involve deficits in Treg function can be studied to develop a global idea of the impact of partial or complete deficiency in a specific molecular mechanism involved in Treg function. In patients with reduced Treg numbers (but no functional deficiency), the expansion of autologous Treg cells could be a suitable therapeutic approach: either infusion of in-vitro autologous expanded cells, infusion of interleukin (IL)-2/anti-IL-2 complex, or both. Treg biology-based therapies may not be suitable in patients with deficits of Treg function, unless their deficit can be corrected in vivo/in vitro. Finally, it is critical to consider the appropriate stage of autoimmune diseases at which administration of Treg cellular therapy can be most effective. We discuss conflicting data regarding whether Treg cells are more effectual at preventing the initiation of autoimmunity, ameliorating disease progression or curing autoimmunity itself.
Keywords: T regulatory cells; Treg; autoimmune; cell therapy; inflammation.
© 2019 British Society for Immunology.
Conflict of interest statement
None declared.
Figures


References
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133:775–87. - PubMed
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151–64. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057–61. - PubMed
-
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4:337–42. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330–6. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

