Developing OECD test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data
- PMID: 30831158
- PMCID: PMC6486396
- DOI: 10.1016/j.yrtph.2019.02.008
Developing OECD test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data
Abstract
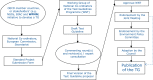
The OECD Working Party on Manufactured Nanomaterials (WPMN) provides a global forum for discussion of nano-safety issues. Together with the OECD Test Guidelines Programme (TGP) the WPMN has explored the need for adaptation of some of the existing OECD Test Guidelines (TGs) and Guidance Documents (GDs) as well as developing new TGs and GDs to specifically address NM issues. An overview is provided of progress in the TGP and WPMN, and information on supporting initiatives, regarding the development of TGs for nanomaterials addressing Physical Chemical Properties, Effects on Biotic Systems, Environmental Fate and Behaviour, and Health Effects. Three TGs specifically addressing manufactured nanomaterials have been adopted: a new TG318 ″Dispersion Stability of Nanomaterials in Simulated Environmental Media", and adaptation of TG412 and TG413 on Subacute Inhalation Toxicity: 28-Day Study/90-day Study. The associated GD39 on Inhalation Toxicity Testing has also been revised. The TGP current develops four new TGs and four GDs. One new TG and six GDs are developed in the WPMN. Six new proposals were submitted to the TGP in 2018. Furthermore, as TGs are accompanied by OECD harmonised templates (OHTs) for data collection, an outline of recently developed OHTs particularly relevant for NMs is also included.
Keywords: Hazard assessment; Nanomaterial; OECD test guidelines; Test methods.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Baun A., Sayre P., Steinhäuser K.G., Rose J. Regulatory relevant and reliable methods and data for determining the environmental fate of manufactured nanomaterials. NanoImpact. 2017;8:1–10.
-
- Drasler B., Sayre P., Steinhäuser K.G., Petri-Fink A., Rothen-Rutishauser B. In vitro approaches to assess the hazard of nanomaterials. NanoImpact. 2017;8:99–116. https://www.sciencedirect.com/science/article/pii/S2452074817300459
-
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). OJ No. L396, 30.12.2006.
-
- EU, European Union, Commission Regulation (EU) 2018/1881 of 03 December 2018 Amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Annexes I, III,VI, VII, VIII, IX, X, XI, and XII to Address Nanoforms of Substances. OJ L 308 of 04 December 2018.
-
- European Commission, Commission Staff Working Paper. SWD(2012) 288 Final of 3.10.2012. Types and Uses of Nanomaterials, Including Safety Aspects, Accompanying the Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee on the Second Regulatory Review on Nanomaterials.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

