Usher syndrome and non-syndromic deafness: Functions of different whirlin isoforms in the cochlea, vestibular organs, and retina
- PMID: 30831381
- PMCID: PMC6474673
- DOI: 10.1016/j.heares.2019.02.007
Usher syndrome and non-syndromic deafness: Functions of different whirlin isoforms in the cochlea, vestibular organs, and retina
Abstract
Usher syndrome (USH) is the leading cause of inherited combined vision and hearing loss. However, mutations in most USH causative genes lead to other diseases, such as hearing loss only or vision loss only. The molecular mechanisms underlying the variable disease manifestations associated with USH gene mutations are unclear. This review focuses on an USH type 2 (USH2) gene encoding whirlin (WHRN; previously known as DFNB31), mutations in which have been found to cause either USH2 subtype USH2D or autosomal recessive non-syndromic deafness type 31 (DFNB31). This review summarizes the current knowledge about different whirlin isoforms encoded by WHRN orthologs in animal models, the interactions of different whirlin isoforms with their partners, and the function of whirlin isoforms in different cellular and subcellular locations. The recent findings regarding the function of whirlin isoforms suggest that disruption of different isoforms may be one of the mechanisms underlying the variable disease manifestations caused by USH gene mutations. This review also presents recent findings about the vestibular defects in Whrn mutant mouse models, which suggests that previous assumptions about the normal vestibular function of USH2 patients need to be re-evaluated. Finally, this review describes recent progress in developing therapeutics for diseases caused by WHRN mutations.
Keywords: Ankle link; Hair cell; Hearing loss; Photoreceptor; Retinitis pigmentosa; Stereocilia; Usher syndrome.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declarations of interest: none
Figures

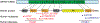



References
-
- Audo I, Bujakowska K, Mohand-Said S, Tronche S, Lancelot ME, Antonio A, Germain A, Lonjou C, Carpentier W, Sahel JA, Bhattacharya S, Zeitz C 2011. A novel DFNB31 mutation associated with Usher type 2 syndrome showing variable degrees of auditory loss in a consanguineous Portuguese family. Mol. Vis 17, 1598–606. - PMC - PubMed
-
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB 2005. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol 7, 148–56. - PubMed
-
- Besnard T, Vache C, Baux D, Larrieu L, Abadie C, Blanchet C, Odent S, Blanchet P, Calvas P, Hamel C, Dollfus H, Lina-Granade G, Lespinasse J, David A, Isidor B, Morin G, Malcolm S, Tuffery-Giraud S, Claustres M, Roux AF 2012. Non-USH2A mutations in USH2 patients. Hum. Mutat 33, 504–10. - PubMed
-
- Boughman JA, Vernon M, Shaver KA 1983. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J. Chronic Dis 36, 595–603. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

