RNA-Based Therapy Utilizing Oculopharyngeal Muscular Dystrophy Transcript Knockdown and Replacement
- PMID: 30831428
- PMCID: PMC6403420
- DOI: 10.1016/j.omtn.2019.02.003
RNA-Based Therapy Utilizing Oculopharyngeal Muscular Dystrophy Transcript Knockdown and Replacement
Abstract
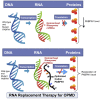
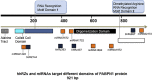
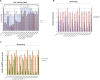
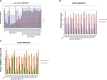
Oculopharyngeal muscular dystrophy (OPMD) is caused by a small expansion of a short polyalanine (polyAla) tract in the poly(A)-binding protein nuclear 1 protein (PABPN1). Despite the monogenic nature of OPMD, no treatment is currently available. Here we report an RNA replacement strategy that has therapeutic potential in cell and C. elegans OPMD models. We develop selective microRNAs (miRNAs) against PABPN1, and we report that miRNAs and our previously developed hammerhead ribozymes (hhRzs) are capable of reducing the expression of both the mRNA and protein levels of PABPN1 by as much as 90%. Since OPMD derives from a very small expansion of GCG within the polyAla tract, our hhRz and miRNA molecules cannot distinguish between the wild-type and mutant mRNAs of PABPN1. Therefore, we designed an optimized-codon wild-type PABPN1 (opt-PABPN1) that is resistant to cleavage by hhRzs and miRNAs. Co-expression of opt-PABPN1 with either our hhRzs or miRNAs restored the level of PABPN1, concomitantly with a reduction in expanded PABPN1-associated cell death in a stable C2C12 OPMD model. Interestingly, knockdown of the PABPN1 by selective hhRzs in the C. elegans OPMD model significantly improved the motility of the PABPN1-13Ala worms. Taken together, RNA replacement therapy represents an exciting approach for OPMD treatment.
Keywords: OPMD; PABPN1; RNA replacement therapy; microRNAs; polyalanine disorders; ribozymes.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Dion P., Shanmugam V., Gaspar C., Messaed C., Meijer I., Toulouse A., Laganiere J., Roussel J., Rochefort D., Laganiere S. Transgenic expression of an expanded (GCG)13 repeat PABPN1 leads to weakness and coordination defects in mice. Neurobiol. Dis. 2005;18:528–536. - PubMed
-
- Shanmugam V., Dion P., Rochefort D., Laganière J., Brais B., Rouleau G.A. PABP2 polyalanine tract expansion causes intranuclear inclusions in oculopharyngeal muscular dystrophy. Ann. Neurol. 2000;48:798–802. - PubMed
-
- Tomé F.M., Fardeau M. Nuclear inclusions in oculopharyngeal dystrophy. Acta Neuropathol. 1980;49:85–87. - PubMed
-
- Brais B., Bouchard J.P., Xie Y.G., Rochefort D.L., Chrétien N., Tomé F.M., Lafrenière R.G., Rommens J.M., Uyama E., Nohira O. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat. Genet. 1998;18:164–167. - PubMed
-
- Brais B., Xie Y.G., Sanson M., Morgan K., Weissenbach J., Korczyn A.D., Blumen S.C., Fardeau M., Tomé F.M., Bouchard J.P. The oculopharyngeal muscular dystrophy locus maps to the region of the cardiac alpha and beta myosin heavy chain genes on chromosome 14q11.2-q13. Hum. Mol. Genet. 1995;4:429–434. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

