The neoepitope landscape of breast cancer: implications for immunotherapy
- PMID: 30832597
- PMCID: PMC6399957
- DOI: 10.1186/s12885-019-5402-1
The neoepitope landscape of breast cancer: implications for immunotherapy
Abstract
Background: Cancer immunotherapy with immune checkpoint blockade (CKB) is now standard of care for multiple cancers. The clinical response to CKB is associated with T cell immunity targeting cancer-induced mutations that generate novel HLA-binding epitopes (neoepitopes).
Methods: Here, we developed a rapid bioinformatics pipeline and filtering strategy, EpitopeHunter, to identify and prioritize clinically relevant neoepitopes from the landscape of somatic mutations. We used the pipeline to determine the frequency of neoepitopes from the TCGA dataset of invasive breast cancers. We predicted HLA class I-binding neoepitopes for 870 breast cancer samples and filtered the neoepitopes based on tumor transcript abundance.
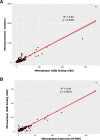
Results: We found that the total mutational burden (TMB) was highest for triple-negative breast cancer, TNBC, (median = 63 mutations, range: 2-765); followed by HER-2(+) (median = 39 mutations, range: 1-1206); and lowest for ER/PR(+)HER-2(-) (median = 32 mutations, range: 1-2860). 40% of the nonsynonymous mutations led to the generation of predicted neoepitopes. The neoepitope load (NEL) is highly correlated with the mutational burden (R2 = 0.86).
Conclusions: Only half (51%) of the predicted neoepitopes are expressed at the RNA level (FPKM≥2), indicating the importance of assessing whether neoepitopes are transcribed. However, of all patients, 93% have at least one expressed predicted neoepitope, indicating that most breast cancer patients have the potential for neo-epitope targeted immunotherapy.
Keywords: Breast cancer; Epitopes; Immunotherapy; Mutation burden; Neoepitope prediction; TNBC.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

