Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer
- PMID: 30832606
- PMCID: PMC6399859
- DOI: 10.1186/s12885-019-5380-3
Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer
Abstract
Background: Treatment options for advanced thyroid cancer refractory to standard therapies are limited. The safety and efficacy of pembrolizumab were evaluated in patients with advanced differentiated thyroid cancer expressing programmed death ligand 1 (PD-L1).
Methods: Patients with advanced thyroid cancer were enrolled in the nonrandomized, phase Ib KEYNOTE-028 trial conducted to evaluate safety and antitumor activity of the anti-programmed death 1 (PD-1) antibody pembrolizumab in advanced solid tumors. Key eligibility criteria were advanced papillary or follicular thyroid cancer, failure of standard therapy, and PD-L1 expression in tumor or stroma cells (assessed by immunohistochemistry). Pembrolizumab 10 mg/kg was administered every 2 weeks up to 24 months or until confirmed progression or intolerable toxicity. The primary endpoint was objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors, version 1.1.
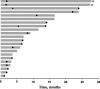
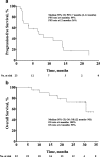
Results: Twenty-two patients were enrolled: median age was 61 years; 59% were women; and 68% had papillary carcinoma. Median follow-up was 31 months (range, 7-34 months). Treatment-related adverse events were observed in 18 (82%) patients; those occurring in ≥15% of patients were diarrhea (n = 7) and fatigue (n = 4). One grade ≥ 3 treatment-related adverse event occurred (colitis, grade 3); no treatment-related discontinuations or deaths occurred. Two patients had confirmed partial response, for an ORR of 9% (95% confidence interval [CI], 1-29%); response duration was 8 and 20 months. Median progression-free survival was 7 months (95% CI, 2-14 months); median overall survival was not reached (95% CI, 22 months to not reached).
Conclusions: Results of this phase Ib proof-of-concept study suggest that pembrolizumab has a manageable safety profile and demonstrate evidence of antitumor activity in advanced differentiated thyroid cancer in a minority of patients treated. Further analyses are necessary to confirm these findings.
Trial registration: Clinicaltrials.gov identifier: NCT02054806 . Registered 4 February 2014.
Keywords: Anti–PD-1; Immunotherapy; PD-1; PD-L1; Pembrolizumab; Thyroid cancer.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol and all amendments were approved by the institutional review board or ethics committee of each participating site (Additional file 1: Table S1) and were conducted per the principles expressed in the Declaration of Helsinki. All patients provided written informed consent to participate.
Consent for publication
Not applicable.
Competing interests
JMM: Consulting or Advisory Role: Merck, EMD Serono, Genentech. MSB: Research Funding: Merck (institution). AP: Research Funding: Merck (institution). TD: Research Funding: Taiho, Novartis, Merck Serono, Astellas Pharma, Merck, Janssen, Boehringer Ingelheim, Takeda, Pfizer, Lilly Sumitomo Group, Chugai Pharma, Bayer, Kyowa, Hakko Kirin, Daiichi Sankyo, Celgene, Amgen. SAP-P: Research Funding: AbbVie, Inc., Aminex Therapeutics, BioMarin Pharmaceutical, Inc., Bristol Myers Squib; Curis, Inc., Five Prime Therapeutics, Genmab, GlaxoSmithKline, Helix BioPharma Corp., Incyte Corp., Medivation, Inc., Merck Sharp and Dohme Corp., Novartis Pharmaceuticals, Pieris Pharmaceuticals, Inc., Pfizer, Puma Biotechnology, Inc., Taiho Oncology, Tesaro, Inc., TransThera Bio, XuanZhu Pharma Co, Ltd., Principia Biopharma, Inc., Cerulean Pharma, Inc., Medimmune, LLC, Boehringer Ingelheim, Jacobio Pharmaceuticals Co, Ltd. SS: Employment: Merck Sharp & Dohme Corp., a subsidiary of Merck and Co., Inc. PT: Employment (at the time of analysis): Merck Sharp & Dohme Corp., a subsidiary of Merck and Co., Inc.; Current employment: Regeneron; Stockholder: MannKind Corporation and Regeneron. JC: Employment: Merck Sharp & Dohme Corp., a subsidiary of Merck and Co., Inc. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- National Cancer Institute: SEER Stat Fact Sheets: Thyroid Cancer. In.; 2016.
-
- National Comprehensive Cancer Network I: NCCN clinical practice guidelines in oncology - thyroid carcinoma v1.2016. In. Edited by 1.2016; 2016.

