Dopamine neuronal protection in the mouse Substantia nigra by GHSR is independent of electric activity
- PMID: 30833218
- PMCID: PMC6531791
- DOI: 10.1016/j.molmet.2019.02.005
Dopamine neuronal protection in the mouse Substantia nigra by GHSR is independent of electric activity
Abstract
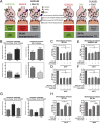
Objective: Dopamine neurons in the Substantia nigra (SN) play crucial roles in control of voluntary movement. Extensive degeneration of this neuronal population is the cause of Parkinson's disease (PD). Many factors have been linked to SN DA neuronal survival, including neuronal pacemaker activity (responsible for maintaining basal firing and DA tone) and mitochondrial function. Dln-101, a naturally occurring splice variant of the human ghrelin gene, targets the ghrelin receptor (GHSR) present in the SN DA cells. Ghrelin activation of GHSR has been shown to protect SN DA neurons against 1-methyl-4-phenyl-1,2,5,6 tetrahydropyridine (MPTP) treatment. We decided to compare the actions of Dln-101 with ghrelin and identify the mechanisms associated with neuronal survival.
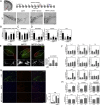
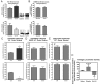
Methods: Histologial, biochemical, and behavioral parameters were used to evaluate neuroprotection. Inflammation and redox balance of SN DA cells were evaluated using histologial and real-time PCR analysis. Designer Receptors Exclusively Activated by Designer Drugs (DREADD) technology was used to modulate SN DA neuron electrical activity and associated survival. Mitochondrial dynamics in SN DA cells was evaluated using electron microscopy data.
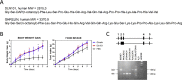
Results: Here, we report that the human isoform displays an equivalent neuroprotective factor. However, while exogenous administration of mouse ghrelin electrically activates SN DA neurons increasing dopamine output, as well as locomotion, the human isoform significantly suppressed dopamine output, with an associated decrease in animal motor behavior. Investigating the mechanisms by which GHSR mediates neuroprotection, we found that dopamine cell-selective control of electrical activity is neither sufficient nor necessary to promote SN DA neuron survival, including that associated with GHSR activation. We found that Dln101 pre-treatment diminished MPTP-induced mitochondrial aberrations in SN DA neurons and that the effect of Dln101 to protect dopamine cells was dependent on mitofusin 2, a protein involved in the process of mitochondrial fusion and tethering of the mitochondria to the endoplasmic reticulum.
Conclusions: Taken together, these observations unmasked a complex role of GHSR in dopamine neuronal protection independent on electric activity of these cells and revealed a crucial role for mitochondrial dynamics in some aspects of this process.
Keywords: Dopamine neuron; GHSR; Mitochondrial dynamics; Mitofusin 2; Parkinson's.
Copyright © 2019. Published by Elsevier GmbH.
Figures



References
-
- Dorsey E.R., Constantinescu R., Thompson J.P., Biglan K.M., Holloway R.G., Kieburtz K. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. - PubMed
-
- Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. Journal of the Neurological Sciences. 1973;20(4):415–455. - PubMed
-
- Carlsson A. Thirty years of dopamine research. Advances in Neurology. 1993;60:1–10. - PubMed
-
- Hornykiewicz O. Parkinson's disease and the adaptive capacity of the nigrostriatal dopamine system: possible neurochemical mechanisms. Advances in Neurology. 1993;60:140–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

