Dual Gene Expression Analysis Identifies Factors Associated with Staphylococcus aureus Virulence in Diabetic Mice
- PMID: 30833333
- PMCID: PMC6479027
- DOI: 10.1128/IAI.00163-19
Dual Gene Expression Analysis Identifies Factors Associated with Staphylococcus aureus Virulence in Diabetic Mice
Abstract
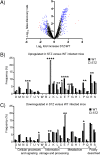
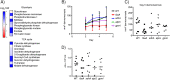
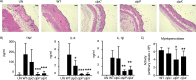
Staphylococcus aureus is a major human pathogen of the skin. The global burden of diabetes is high, with S. aureus being a major complication of diabetic wound infections. We investigated how the diabetic environment influences S. aureus skin infection and observed an increased susceptibility to infection in mouse models of both type I and type II diabetes. A dual gene expression approach was taken to investigate transcriptional alterations in both the host and bacterium after infection. While analysis of the host response revealed only minor changes between infected control and diabetic mice, we observed that S. aureus isolated from diabetic mice had significant increases in the levels of genes associated with translation and posttranslational modification and chaperones and reductions in the levels of genes associated with amino acid transport and metabolism. One family of genes upregulated in S. aureus isolated from diabetic lesions encoded the Clp proteases, associated with the misfolded protein response. The Clp proteases were found to be partially glucose regulated as well as influencing the hemolytic activity of S. aureus Strains lacking the Clp proteases ClpX, ClpC, and ClpP were significantly attenuated in our animal model of skin infection, with significant reductions observed in dermonecrosis and bacterial burden. In particular, mutations in clpP and clpX were significantly attenuated and remained attenuated in both normal and diabetic mice. Our data suggest that the diabetic environment also causes changes to occur in invading pathogens, and one of these virulence determinants is the Clp protease system.
Keywords: Clp protease; Staphylococcus aureus; diabetes; host-pathogen interactions.
Copyright © 2019 American Society for Microbiology.
Figures




References
-
- CDC. 2014. National diabetes statistics report. CDC, Atlanta, GA.
-
- Lepper PM, Ott S, Nuesch E, von Eynatten M, Schumann C, Pletz MW, Mealing NM, Welte T, Bauer TT, Suttorp N, Juni P, Bals R, Rohde G, German Community Acquired Pneumonia Competence Network . 2012. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ 344:e3397. doi: 10.1136/bmj.e3397. - DOI - PMC - PubMed
-
- Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J, Emerging Risk Factors Collaboration . 2011. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364:829–841. doi: 10.1056/NEJMoa1008862. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

