The Mouse Inhalation Model of Cryptococcus neoformans Infection Recapitulates Strain Virulence in Humans and Shows that Closely Related Strains Can Possess Differential Virulence
- PMID: 30833336
- PMCID: PMC6479045
- DOI: 10.1128/IAI.00046-19
The Mouse Inhalation Model of Cryptococcus neoformans Infection Recapitulates Strain Virulence in Humans and Shows that Closely Related Strains Can Possess Differential Virulence
Abstract
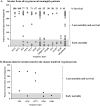
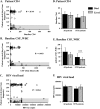
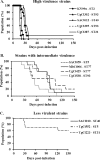
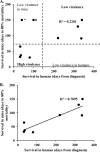
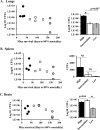
Cryptococcal meningitis (CM) causes high rates of HIV-related mortality, yet the Cryptococcus factors influencing patient outcome are not well understood. Pathogen-specific traits, such as the strain genotype and degree of antigen shedding, are associated with the clinical outcome, but the underlying biology remains elusive. In this study, we examined factors determining disease outcome in HIV-infected cryptococcal meningitis patients infected with Cryptococcus neoformans strains with the same multilocus sequence type (MLST). Both patient mortality and survival were observed during infections with the same sequence type. Disease outcome was not associated with the patient CD4 count. Patient mortality was associated with higher cryptococcal antigen levels, the cerebrospinal fluid (CSF) fungal burden by quantitative culture, and low CSF fungal clearance. The virulence of a subset of clinical strains with the same sequence type was analyzed using a mouse inhalation model of cryptococcosis. We showed a strong association between human and mouse mortality rates, demonstrating that the mouse inhalation model recapitulates human infection. Similar to human infection, the ability to multiply in vivo, demonstrated by a high fungal burden in lung and brain tissues, was associated with mouse mortality. Mouse survival time was not associated with single C. neoformans virulence factors in vitro or in vivo; rather, a trend in survival time correlated with a suite of traits. These observations show that MLST-derived genotype similarities between C. neoformans strains do not necessarily translate into similar virulence either in the mouse model or in human patients. In addition, our results show that in vitro assays do not fully reproduce in vivo conditions that influence C. neoformans virulence.
Keywords: Cryptococcus; Cryptococcus neoformans; cryptococcosis; human; meningitis; model; mouse; pathogenesis; sequence type; virulence.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, Harrison T. 2007. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 45:76–80. doi: 10.1086/518607. - DOI - PubMed
-
- Wiesner DL, Moskalenko O, Corcoran JM, McDonald T, Rolfes MA, Meya DB, Kajumbula H, Kambugu A, Bohjanen PR, Knight JF, Boulware DR, Nielsen K. 2012. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. mBio 3:e00196-12. doi: 10.1128/mBio.00196-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

