Metabolites Involved in Immune Evasion by Batrachochytrium dendrobatidis Include the Polyamine Spermidine
- PMID: 30833338
- PMCID: PMC6479046
- DOI: 10.1128/IAI.00035-19
Metabolites Involved in Immune Evasion by Batrachochytrium dendrobatidis Include the Polyamine Spermidine
Abstract
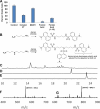
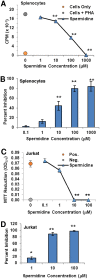
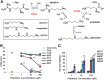
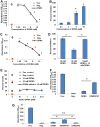
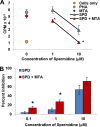
Amphibians have been declining around the world for more than four decades. One recognized driver of these declines is the chytrid fungus Batrachochytrium dendrobatidis, which causes the disease chytridiomycosis. Amphibians have complex and varied immune defenses against B. dendrobatidis, but the fungus also has a number of counterdefenses. Previously, we identified two small molecules produced by the fungus that inhibit frog lymphocyte proliferation, methylthioadenosine (MTA) and kynurenine (KYN). Here, we report on the isolation and identification of the polyamine spermidine (SPD) as another significant immunomodulatory molecule produced by B. dendrobatidis SPD and its precursor, putrescine (PUT), are the major polyamines detected, and SPD is required for growth. The major pathway of biosynthesis is from ornithine through putrescine to spermidine. An alternative pathway from arginine to agmatine to putrescine appears to be absent. SPD is inhibitory at concentrations of ≥10 μM and is found at concentrations between 1 and 10 μM in active fungal supernatants. Although PUT is detected in the fungal supernatants, it is not inhibitory to lymphocytes even at concentrations as high as 100 μM. Two other related polyamines, norspermidine (NSP) and spermine (SPM), also inhibit amphibian lymphocyte proliferation, but a third polyamine, cadaverine (CAD), does not. A suboptimal (noninhibitory) concentration of MTA (10 μM), a by-product of spermidine synthesis, enhances the inhibition of SPD at 1 and 10 μM. We interpret these results to suggest that B. dendrobatidis produces an "armamentarium" of small molecules that, alone or in concert, may help it to evade clearance by the amphibian immune system.
Keywords: Batrachochytrium dendrobatidis; amphibian declines; difluoromethylarginine (DFMA); difluoromethylornithine (DFMO); immunomodulation; methylthioadenosine; polyamine; putrescine; spermidine; splenocytes.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- International Union for Conservation of Nature and Natural Resources. 2018. Red list of threatened species. http://www.iucnredlist.org/. Accessed 14 November 2018.
-
- Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227. doi:10.2307/3761366. - DOI
-
- Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F, Pasmans F. 2013. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci U S A 110:15325–15329. doi:10.1073/pnas.1307356110. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

