A Dual-Species Biofilm with Emergent Mechanical and Protective Properties
- PMID: 30833350
- PMCID: PMC6707914
- DOI: 10.1128/JB.00670-18
A Dual-Species Biofilm with Emergent Mechanical and Protective Properties
Abstract
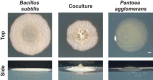
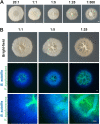
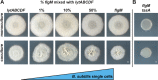
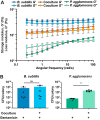
Many microbes coexist within biofilms, or multispecies communities of cells encased in an extracellular matrix. However, little is known about the microbe-microbe interactions relevant for creating these structures. In this study, we explored a striking dual-species biofilm between Bacillus subtilis and Pantoea agglomerans that exhibited characteristics that were not predictable from previous work examining monoculture biofilms. Coculture wrinkle formation required a P. agglomerans exopolysaccharide as well as the B. subtilis amyloid-like protein TasA. Unexpectedly, other B. subtilis matrix components essential for monoculture biofilm formation were not necessary for coculture wrinkling (e.g., the exopolysaccharide EPS, the hydrophobin BslA, and cell chaining). In addition, B. subtilis cell chaining prevented coculture wrinkling, even though chaining was previously associated with more robust monoculture biofilms. We also observed that increasing the relative proportion of P. agglomerans (which forms completely featureless monoculture colonies) increased coculture wrinkling. Using microscopy and rheology, we observed that these two bacteria assemble into an organized layered structure that reflects the physical properties of both monocultures. This partitioning into distinct regions negatively affected the survival of P. agglomerans while also serving as a protective mechanism in the presence of antibiotic stress. Taken together, these data indicate that studying cocultures is a productive avenue to identify novel mechanisms that drive the formation of structured microbial communities.IMPORTANCE In the environment, many microbes form biofilms. However, the interspecies interactions underlying bacterial coexistence within these biofilms remain understudied. Here, we mimic environmentally relevant biofilms by studying a dual-species biofilm formed between Bacillus subtilis and Pantoea agglomerans and subjecting the coculture to chemical and physical stressors that it may experience in the natural world. We determined that both bacteria contribute structural elements to the coculture, which is reflected in its overall viscoelastic behavior. Existence within the coculture can be either beneficial or detrimental depending on the context. Many of the features and determinants of the coculture biofilm appear distinct from those identified in monoculture biofilm studies, highlighting the importance of characterizing multispecies consortia to understand naturally occurring bacterial interactions.
Keywords: Bacillus subtilis; Pantoea agglomerans; antibiotic stress; biofilm; cell-cell interactions; coculture; dual-species; rheology; wrinkles.
Copyright © 2019 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

