AAV cis-regulatory sequences are correlated with ocular toxicity
- PMID: 30833387
- PMCID: PMC6431174
- DOI: 10.1073/pnas.1821000116
AAV cis-regulatory sequences are correlated with ocular toxicity
Abstract
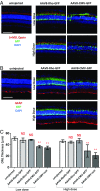
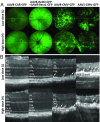
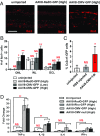
Adeno-associated viral vectors (AAVs) have become popular for gene therapy, given their many advantages, including their reduced inflammatory profile compared with that of other viruses. However, even in areas of immune privilege such as the eye, AAV vectors are capable of eliciting host-cell responses. To investigate the effects of such responses on several ocular cell types, we tested multiple AAV genome structures and capsid types using subretinal injections in mice. Assays of morphology, inflammation, and physiology were performed. Pathological effects on photoreceptors and the retinal pigment epithelium (RPE) were observed. Müller glia and microglia were activated, and the proinflammatory cytokines TNF-α and IL-1β were up-regulated. There was a strong correlation between cis-regulatory sequences and toxicity. AAVs with any one of three broadly active promoters, or an RPE-specific promoter, were toxic, while AAVs with four different photoreceptor-specific promoters were not toxic at the highest doses tested. There was little correlation between toxicity and transgene, capsid type, preparation method, or cellular contaminants within a preparation. The toxic effect was dose-dependent, with the RPE being more sensitive than photoreceptors. Our results suggest that ocular AAV toxicity is associated with certain AAV cis-regulatory sequences and/or their activity and that retinal damage occurs due to responses by the RPE and/or microglia. By applying multiple, sensitive assays of toxicity, AAV vectors can be designed so that they can be used safely at high dose, potentially providing greater therapeutic efficacy.
Keywords: AAV; gene therapy; retina; retinal pigment epithelium; toxicity.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: The authors note we have received funding from Astellas for our work.
Figures



References
-
- Dalkara D, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra76. - PubMed
-
- Bainbridge JWB, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

