Generating Robust and Informative Nonclinical In Vitro and In Vivo Bacterial Infection Model Efficacy Data To Support Translation to Humans
- PMID: 30833428
- PMCID: PMC6496039
- DOI: 10.1128/AAC.02307-18
Generating Robust and Informative Nonclinical In Vitro and In Vivo Bacterial Infection Model Efficacy Data To Support Translation to Humans
Abstract
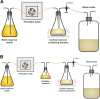
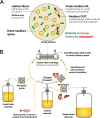
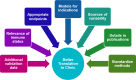
In June 2017, the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, organized a workshop entitled "Pharmacokinetics-Pharmacodynamics (PK/PD) for Development of Therapeutics against Bacterial Pathogens." The aims were to discuss details of various PK/PD models and identify sound practices for deriving and utilizing PK/PD relationships to design optimal dosage regimens for patients. Workshop participants encompassed individuals from academia, industry, and government, including the United States Food and Drug Administration. This and the accompanying review on clinical PK/PD summarize the workshop discussions and recommendations. Nonclinical PK/PD models play a critical role in designing human dosage regimens and are essential tools for drug development. These include in vitro and in vivo efficacy models that provide valuable and complementary information for dose selection and translation from the laboratory to human. It is crucial that studies be designed, conducted, and interpreted appropriately. For antibacterial PK/PD, extensive published data and expertise are available. These have been leveraged to develop recommendations, identify common pitfalls, and describe the applications, strengths, and limitations of various nonclinical infection models and translational approaches. Despite these robust tools and published guidance, characterizing nonclinical PK/PD relationships may not be straightforward, especially for a new drug or new class. Antimicrobial PK/PD is an evolving discipline that needs to adapt to future research and development needs. Open communication between academia, pharmaceutical industry, government, and regulatory bodies is essential to share perspectives and collectively solve future challenges.
Keywords: best practices; drug development; hollow fiber system; in vitro infection models; mouse infection models; optimal design; pharmacokinetics/pharmacodynamics; progression and decision criteria; validation; workshop summary.
Copyright © 2019 Bulitta et al.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

