Adverse Drug Reactions in an Oncological Population: Prevalence, Predictability, and Preventability
- PMID: 30833488
- PMCID: PMC6738308
- DOI: 10.1634/theoncologist.2018-0476
Adverse Drug Reactions in an Oncological Population: Prevalence, Predictability, and Preventability
Abstract
Background: Our goal was to determine (a) the prevalence of multimorbidity and polypharmacy in patients with cancer and (b) the prevalence, predictability, and preventability of adverse drug reactions (ADRs) causing/contributing to hospitalization.
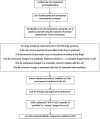
Materials and methods: We conducted a 12-month prospective observational study of patients aged ≥16 years admitted to an oncology center. Older adults were aged ≥70 years.
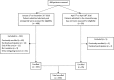
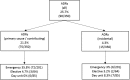
Results: We enrolled 350 patients: 52.3% (n = 183) female, mean age 63.6 years (SD 12.1), 36.6% (n = 121) aged ≥70 years. Multimorbidity (≥2 conditions) was identified in 96.9%; 68% had ≥5 conditions. The median number of medications was 6 (interquartile range [IQR] 4-8); 47% were prescribed ≥6 medications and 11.4% ≥11 medications. Older adults had higher numbers of comorbid conditions (7 [IQR 5-10] vs. 5 [IQR 3-7]) and were prescribed more medications (median 7 [IQR 4-9] vs. 4 [IQR 2-7]). ADRs caused/contributed to hospitalization in 21.5% (n = 75): 35.8% (n = 72) of emergency admissions and 4.7% (n = 3) of elective admissions. The most common ADRs were neutropenia with infection (25.3%), dyspepsia/nausea/vomiting (20%), and constipation (20%). Causative medications included systemic anticancer therapies (SACTs; 53.3%), opioids (17.3%), corticosteroids (6.7%), and nonsteroidal anti-inflammatory drugs (5.3%). ADR prevalence was similar in older and younger adults secondary to SACTs (8.3% vs. 13.1%), non-cancer medications (10.7% vs. 8.3%), and both (0% vs. 1.3%). ADRs were predictable in 89.3% (n = 67), definitely avoidable in 29.3% (n = 22), and possibly avoidable in 33.3% (n = 25). No association was identified between ADRs and age, gender, daily medication number, length of stay, or death. No ADR predictor variables were identified by logistic regression.
Conclusion: More than 21% of admissions to an oncology service are ADR-related. ADRs are caused by both SACTs and non-cancer-specific medications. The majority are predictable; ≥60% may be preventable. Patients with cancer have high levels of multimorbidity and polypharmacy, which require vigilance for related adverse outcomes.
Implications for practice: A diagnosis of cancer often occurs in patients with multimorbidity and polypharmacy. Cancer can cause an altered physiological environment, placing patients at risk of drug-drug interactions, drug-disease interactions, and adverse drug reactions (ADRs). This study identified that ADRs caused or contributed to one in five hospital admissions of patients with cancer. ADRs were caused by systemic anticancer therapies (SACTs) in 53.3% of cases and non-cancer medications in 45.4% of cases, and a combination of both in 1.3%. ADRs occurred in similar frequencies in older and younger patients secondary to SACTs (8.3% vs. 13.1%, p = .295), non-SACTs (10.7% vs. 8.3%, p = .107), and a combination of both (0% vs. 1.3%, p = .240). The majority of ADRs were predictable (89.3%) and potentially preventable (62.6%). These findings support the need for increased awareness of medication-related adversity in patients with cancer and interventions to minimize their occurrence, thus supporting the American Society of Clinical Oncology guidelines that recommend adults ≥65 years of age receiving chemotherapy have geriatric assessment to identify medical and medication issues.
Keywords: Adverse drug reactions; Clinical oncology; Hospitalization; Multimorbidity; Polypharmacy.
© AlphaMed Press 2019.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. - PubMed
-
- Smith BD, Smith GL, Hurria A et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–2765. - PubMed
-
- National Cancer Registry . Cancer projections for Ireland 2015‐2040. Cork, Ireland: National Cancer Registry, 2014.
-
- Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics‐‐2015 update: A report from the American Heart Association. Circulation 2015;131:E29–E322. - PubMed
-
- Finucane C, O'Connell MD, Fan CW et al. Age‐related normative changes in phasic orthostatic blood pressure in a large population study: Findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation 2014;130:1780–1796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

