Spinal Motor Circuit Synaptic Plasticity after Peripheral Nerve Injury Depends on Microglia Activation and a CCR2 Mechanism
- PMID: 30833511
- PMCID: PMC6495126
- DOI: 10.1523/JNEUROSCI.2945-17.2019
Spinal Motor Circuit Synaptic Plasticity after Peripheral Nerve Injury Depends on Microglia Activation and a CCR2 Mechanism
Abstract
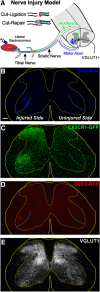
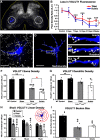
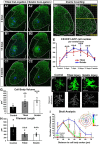
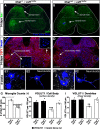
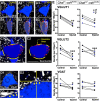
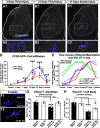
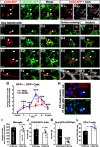
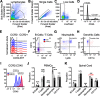
Peripheral nerve injury results in persistent motor deficits, even after the nerve regenerates and muscles are reinnervated. This lack of functional recovery is partly explained by brain and spinal cord circuit alterations triggered by the injury, but the mechanisms are generally unknown. One example of this plasticity is the die-back in the spinal cord ventral horn of the projections of proprioceptive axons mediating the stretch reflex (Ia afferents). Consequently, Ia information about muscle length and dynamics is lost from ventral spinal circuits, degrading motor performance after nerve regeneration. Simultaneously, there is activation of microglia around the central projections of peripherally injured Ia afferents, suggesting a possible causal relationship between neuroinflammation and Ia axon removal. Therefore, we used mice (both sexes) that allow visualization of microglia (CX3CR1-GFP) and infiltrating peripheral myeloid cells (CCR2-RFP) and related changes in these cells to Ia synaptic losses (identified by VGLUT1 content) on retrogradely labeled motoneurons. Microgliosis around axotomized motoneurons starts and peaks within 2 weeks after nerve transection. Thereafter, this region becomes infiltrated by CCR2 cells, and VGLUT1 synapses are lost in parallel. Immunohistochemistry, flow cytometry, and genetic lineage tracing showed that infiltrating CCR2 cells include T cells, dendritic cells, and monocytes, the latter differentiating into tissue macrophages. VGLUT1 synapses were rescued after attenuating the ventral microglial reaction by removal of colony stimulating factor 1 from motoneurons or in CCR2 global KOs. Thus, both activation of ventral microglia and a CCR2-dependent mechanism are necessary for removal of VGLUT1 synapses and alterations in Ia-circuit function following nerve injuries.SIGNIFICANCE STATEMENT Synaptic plasticity and reorganization of essential motor circuits after a peripheral nerve injury can result in permanent motor deficits due to the removal of sensory Ia afferent synapses from the spinal cord ventral horn. Our data link this major circuit change with the neuroinflammatory reaction that occurs inside the spinal cord following injury to peripheral nerves. We describe that both activation of microglia and recruitment into the spinal cord of blood-derived myeloid cells are necessary for motor circuit synaptic plasticity. This study sheds new light into mechanisms that trigger major network plasticity in CNS regions removed from injury sites and that might prevent full recovery of function, even after successful regeneration.
Keywords: CCR2; CX3CR1; VGLUT1; microglia; nerve injury; stretch reflex.
Copyright © 2019 the authors.
Figures
References
-
- Aldskogius H, Arvidsson J, Grant G (1985) The reaction of primary sensory neurons to peripheral nerve injury with particular emphasis on transganglionic changes. Brain Res 357:27–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
